Recurrent nonmassive hemoptysis is one of the common outpatient visits in a pulmonary clinic and, in many cases, the cause of hemoptysis remains unknown. We describe a case of recurrent nonmassive hemoptysis of an unknown cause, which was eventually diagnosed as endobronchial telangiectasia using narrow band imaging during bronchoscopy.
A 37-year-old male patient, an office worker, presented to the pulmonary medicine outpatient with a complaint of recurrent hemoptysis for the last 2 months. He previously had unremarkable rhino-laryngoscopy and blood tests from multiple evaluations, including HIV testing. The hemoptysis was not resolved despite antibiotics and transamine treatment. The frequency of hemoptysis was at least two to three times per month, with fresh and clotted blood (5-10 ml) in each episode (Figure 1).
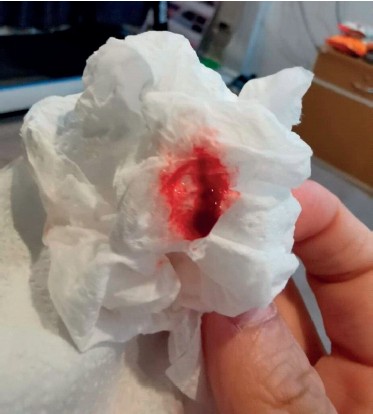
Figure 1: 5-10ml of Fresh and clotted blood from the hemoptysis episode.
Onset was sudden blood-tinged sputum without any obvious precipitating factor. The patient denied any associated symptoms of cough, shortness of breath, fever, and anorexia or weight loss. No other bleeding site was reported. He was a non-smoker, nonalcoholic and had no previous history of tuberculosis, chronic lung or airway disease. He denied history of Raynaud’s phenomenon or any vasculitis syndrome. He denied familial history of bleeding diathesis.
Initial Physical Examination was unremarkable, specifi- cally with no sign of portal hypertension, cutaneous or oral telangiectasis, vasculitis or bleeding diathesis. Initial blood tests for complete blood count and coagulogram were unremarkable. The chest radiograph was unremarkable. Computed tomography (CT) of the chest was unremarkable for endobronchial and parenchymal abnormalities. Given the unclear source of hemoptysis, a diagnostic surveillance Flexible Optic Bronchoscopy (FOB) was performed, which showed telangiectatic lesions in the subglottic area to one-third of the proximal trachea (Figure 2).
The lesions were flat, non-pulsatile, and with a sign of resolved active bleeding, surrounded by normal mucosa. Two-thirds of distal trachea and main and lobar bronchi were normal. Narrow band imaging (NBI) was also performed during FOB, which confirmed the presence of telangiectasia (Figure 3).
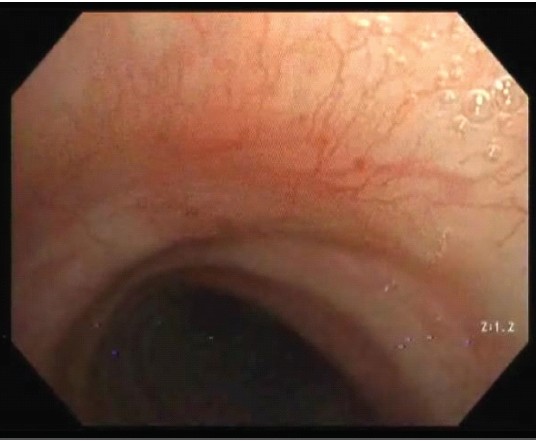
Figure 2: Telangiectatic lesions in the subglottic area to one-third of the proximal trachea.
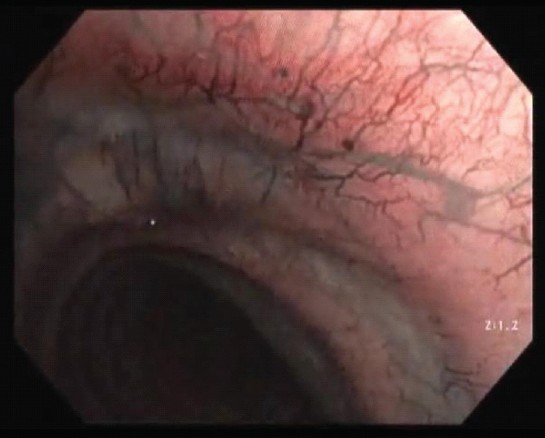
Figure 3: Narrow band imaging (NBI) was also performed during FOB which confirmed the presence of telangiectasia.
The rest of the bronchoscopic examination was unremarkable except for left nasal polyp, which prohibited the passage of the endoscope. Further consultation from the ENT department for repeated rhinolarynscope also showed telangiectasis at left nasopharynx. Subsequently, esophageal gastroscope was unremarkable and no sign of Osler-Weber-Rendu was found. The patient’s unexplained hemoptysis was due to spontaneous bleeding from endobrochial telangeiectasia, which were self- limited. Given the diffuse nature of lesions, no therapeutic procedure was done at present. The patient is currently under follow-up with a plan for therapeutic argon plasma coagulation (APC) application in case of recurrence of hemoptysis.
Endobronchial telangiectasia is a rare case. A patient’s common manifestation is chronic, unexplained hemoptysis. Endobronchial telangiectasia as a cause of hemoptysis has been associated with systemic sclerosis, cirrhosis and hereditary hemorrhagic telangiectasia (HHT).1 Unfortunately, the cause of a few endobronchial telangiectasia cases remains unknown, commonly termed as idiopathic endobronchial telangiectasia. Interestingly, telangiectasia, which originates from the bronchial circulation, will not cause abnormal gas exchange or hypoxia, in contrast to the more common arteriovenous malformations (AVM) of the pulmonary circulation seen in patients with HHT. Endobronchial telangiectasia can lead to massive hemoptysis which may be, on occasions, missed by a casual examination during FOB, and these patients may undergo multiple diagnostic procedures before the diagnosis is established.
Two essential investigations in the evaluation of hemoptysis include CT chest and a flexible bronchoscopy (FOB), in search for an active bleeding site and if any, endobronchial lesion. Usually, CT chest is unremarkable.
In contrast to hemangioma, which usually appears as a smooth-surfaced pedunculated lobular tumor, typical gross findings of ET from FOB reveal multiple flat, red, non-pulsatile areas of tiny superficial vessels, 2 to 4 mm, surrounded by normal mucosa.2 The use of narrow band imaging (NBI) in conjunction with continuous white light (CWL) during bronchoscopy may be used for better identification of superficial vascular abnormalities and telangiectasia lesions.3
NBI is a technology in bronchoscopy that enhances the visibility of vessels of the mucosa and differentiation between inflammation and pathological vascularisation of tumor by optimizing light wavelengths at 415 nm and 540 nm. NBI has found wide applications in gastroenterology and urology. However, its application in bronchoscopy has been limited due to scant literature on the subject. This led to more effective diagnosis and management of the abnormal vessels causing the hemoptysis.4
The therapeutic modalities for endobronchial telangiectasias are interventional bronchoscopic electrocoagulation or argon plasma coagulation (APC). As compared with electrocoagulation, APC has a lower risk of perforation or cartilage damage and better control of depth.5 Surgical resection is reserved for larger lesions not amenable to endovascular or bronchoscopic modalities.
Endobronchial telangiectasia and hemoptysis cases have been report in a limited number of cases. This case demonstrates the benefit of NBI during bronchoscopic examination to identify mucosal vascular abnormalities in patients with unexplained chronic hemoptysis. Using narrow band imaging may aid in the diagnosis of such a rare disease and prompt early definitive treatment for unexplained nonmassive hemoptysis.
The authors have no conflicts of interest or any funding to declare.