A cinetobacter baumannii (A. baumannii) , gram-negative coccobacilli, is a leading cause of nosocomial infection in Thailand.1-4 This pathogen contain multi-mechanisms of resistance; thus the antibiotic susceptibility profile is often resistant to many antimicrobials, including carbapenems (Carbapenem-resistant A. baumannii: CRAB).1-5 Currently, incidences of CRAB infection have increased, resulting in morbidity, mortality and increased healthcare costs.1-3 One contributing factor related to the high mortality rate among patients infected with CRAB is that there is a higher chance to receive inactive antimicrobial agents due to highly resistant antibiotics.1,4,6 Therefore, patients who received combination therapy are more likely to have been given at least one antibiotic to which CRAB was susceptible.4
Combination antimicrobial therapy has been proposed as a treatment option for CRAB infections since therapy improves treatment outcomes and prevents the emergence of drug resistance.7-9 Due to how the majority of CRAB retains to colistin susceptible isolates, colistin is the mainstay in combination with other antimicrobials against CRAB.3,5 Tigecycline, the first drug in the glycylcycline class, has been approved for the treatment of complicated skin and skin-structure infections, complicated intra-abdominal infections and community-acquired pneumonia.10 Tigecycline has potent in vitro activity against a wide range of gram-positive and gram-negative bacteria including Acinetobacter spp.10,11 Since tigecycline can distribute into various tissues and body fluids including lung tissue,10-12 tigecycline is a suitable option therefore to combine with colistin for hospital-acquired pneumonia caused by the CRAB.10,13,14 The data from in vitro studies showed a synergistic rate of colistin plus tigecycline against CRAB strains ranging between 2.0% and 67.4 %.8, 9,15,16 However, antagonistic effects of such a combination might be detected.17 Since the benefit of a combination of colistin and tigecycline is ambiguous, we conducted this particular study to determine in vitro antimicrobial susceptibility and the synergistic activity of colistin in combination with tigecycline against clinical strains of CRAB.
Bacterial strains
All of the clinical CRAB isolates were obtained from patients who were admitted to Phayathai 2 International Hospital, Bangkok between August 2014 and April 2015. Only the first isolate from each patient was allowed for testing. For this study, CRAB was defined as isolates that were resistant to carbapenems (imipenem or meropenem) by a routine disk diffusion method according to the Clinical and Laboratory Standards Institute (CLSI) 2018.18 This study protocol was approved with a waiver for informed consent [No. ID0013/59, issued date].
Determination of minimum inhibitory concentration
The minimum inhibitory concentration (MIC) of colistin (Sigma-Aldrich, St Louis, MO) and tigecycline (Wago Pure Chemical Industry, Osaka, Japan) were determined by broth microdilution using Mueller-Hinton broth II (Difco, Detroit, MI). All tested isolates were incubated at 35°C for 20 hours. The lowest concentration of an antimicrobial agent that inhibited the visible growth of CRAB isolate was defined as the MIC. Pseudomonas aeruginosa ATCC 27853 [Department of Medical Sciences Type (DMST)] culture collection, Bangkok, Thailand] was used as positive control.
Colistin susceptible breakpoints were defined as ≤ 2 μg/mL according to CLSI 2018. Due to the lack of a susceptible breakpoint, we used the pharmacokinetic-pharmacodynamics (PK-PD) breakpoint according to the European Committee on Antimicrobial Susceptibility Testing 2018 (EUCAST). A MIC breakpoint of ≤ 0.25 μg/mL was established for tigecycline. If the MIC was less than or equal to the PK-PD susceptible breakpoint, this suggested that tigecycline could be used.19
The synergistic testing
Checkerboard synergy testing was performed in sterile 96-well U-bottom plates. Each well of columns was filled two-fold with a serial dilution of tigecycline and rows in which each well were filled with a serial dilution of the colistin. The 12-tigecycline concentrations were 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, 16, and 32 μg/mL. The 8-colistin concentrations were 0.5, 1, 2, 3, 4, 6, 8, and 16 μg/mL. Inoculum was added to each well to achieve a final concentration of 1x105 cfu/well. The plates were incubated at 35°C for 20 hours. The MIC was defined as the lowest concentration inhibiting visible growth of bacteria in the well. Fractional inhibitory concentration index (ΣFIC) was calculated from the following equation.
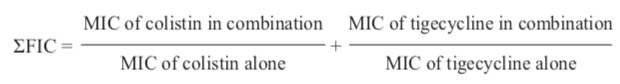
The results of a 4-fold reduction in the MICs compared with the MICs of agents alone are synergistic (ΣFIC ≤ 0.5). Fractional inhibitory concentration indexes in the range of 0.5 to 1.0, > 1.0 to < 4.0 and ≥ 4.0 are considered as additive, indifferent, and antagonistic effects, respectively.
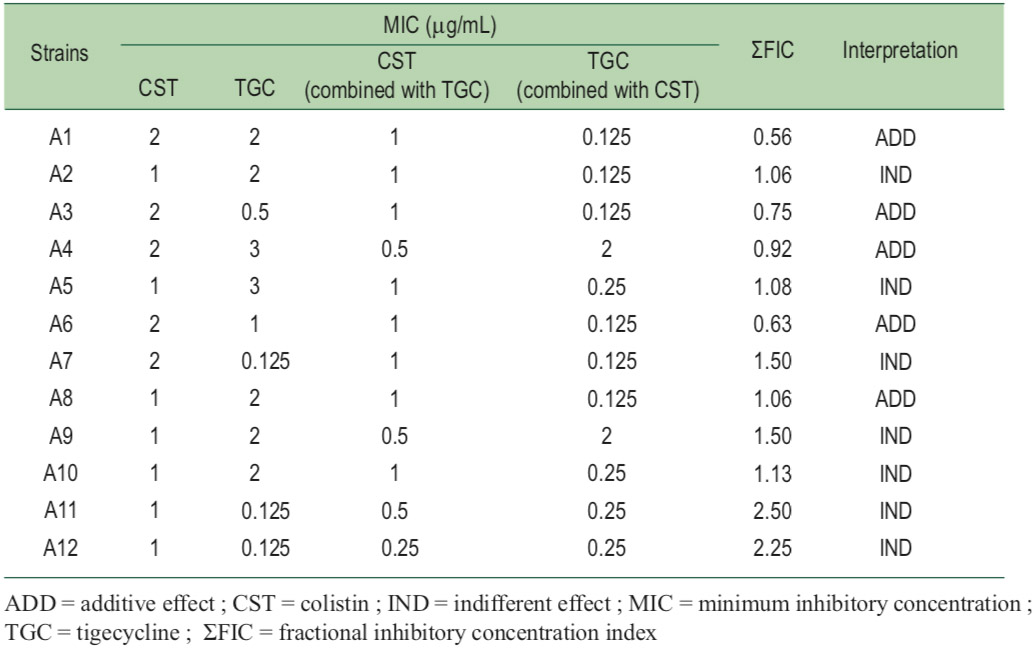
The MICs and synergistic testing of the 12-CRAB isolate are shown in Table 1. All of CRAB isolates were susceptible to colistin with MICs in a range of 1 to 2 μg/mL whereas MICs of tigecycline against CRAB ranged 0.125 to 3.0 μg/mL. Only three out of 12 (25%) CRAB isolates were classified as tige- cycline susceptible strains.With synergistic testing, the MICs of both antimicrobials were decreased in most of the CRAB isolates. In combination, colistin and tigecycline MICs were reduced to 0.25–1.0 μg/mL and 0.125–2.0 μg/mL, respectively. The effects of tigecycline combined with colistin on most of the CRAB isolates reverted to tigecycline susceptible strains (10 out of 12 isolates; 83.3%).
The ΣFICs of colistin combined with tigecycline were in the range of 0.56 to 2.5. The colistin and tigecycline combina- tion revealed additive and indifferent effects in five and seven of the 12-CRAB isolates, respectively. Neither synergism nor antagonism of the combination of colistin and tigecycline was demonstrated in this study.
In the present study, our CRAB isolates were universally susceptible to colistin. Only one-fourth of the CRAB isolates were susceptible to tigecycline, based on PK-PD breakpoints (tigecycline < 0.25 μg/mL). The A. baumannii, for which EUCAST breakpoints have not been determined, was investigated for antimicrobial susceptibility using interpretation based on PK-PD breakpoints and the patient is probably treatable with tigecycline.
This low threshold of the tigecycline-breakpoint is due to a maximum plasma concentration of multiple doses of 50-mg intravenous measuring only 0.63 μg/mL.11 Moreover, pneumonia is the most prominent source infected with CRAB. Mean tigecycline concentrations in the lung epithelial lining fluid (ELF) at each bronchoscopy time point ranged from 0.37 μg/mL at 6 h to 0.06 μg/mL at 24 h.20 Thus, tigecycline mono- therapy for nosocomial pneumonia should be used with cau- tion. This warning was addressed in a meta-analysis study by Arthur et al.21 showing a statistically significant decrease in the clinical cure for tigecycline when compared with imipenem-cilastatin (OR 0.44, 95% CI 0.23 to 0.84). Data from matched cohort analysis also showed a higher mortality rate in MDR A. baumannii pneumonia patients who received tigecycline when compared with colistin groups among those with tigecycline MIC > 2 μg/mL.22 In the setting of extensively drug-resistant (XDR) A. baumannii bacteremia, increased 14- day mortality was associated with colistin-tigecycline therapy in those with a tigecycline MIC greater than 2 μg/mL.23 Thus, the antibiotic combination to reduce the MIC of tigecycline or increase the dose of tigecycline might be a therapeutic option in clinical practice.
Table 1: Minimum inhibitory concentration (MIC) and the synergistic testing of colistin and tigecycline combination by the checkerboard method against 12-clinical carbapenem-resistant A. baumannii isolates.
 In our synergy testing, there were no synergistic effects of colistin combined with tigecycline. Similarly, several previous studies evaluating the in vitro synergistic activity showed minimal effects of colistin combined with tigecycline. Ni et al. reported only a 4.3% synergy rate among multidrug-resistant (MDR) A.baumannii strains by means of the checkerboard method.9 In the same way, Karaoglan et al. and Kaya et al. have determined by the E-test method that a colistin/tigecycline combination displayed synergistic activity in 12% and 2% of MDR A.baumannii strains.15,16
In our synergy testing, there were no synergistic effects of colistin combined with tigecycline. Similarly, several previous studies evaluating the in vitro synergistic activity showed minimal effects of colistin combined with tigecycline. Ni et al. reported only a 4.3% synergy rate among multidrug-resistant (MDR) A.baumannii strains by means of the checkerboard method.9 In the same way, Karaoglan et al. and Kaya et al. have determined by the E-test method that a colistin/tigecycline combination displayed synergistic activity in 12% and 2% of MDR A.baumannii strains.15,16
There were no antagonistic effects in our findings. In contrast, Cikman et al.17 reported 80% antagonistic effects from a colistin/tigecycline combination in CRAB strains. This different outcome might be a result of the method used for synergy testing. Recently, a joint EUCAST and CLSI subcommittee issued recommendations confirming that broth microdilution (BMD) is so far the only valid method for MIC determination and that E-tests have questioned the validity of MICs. Thus, synergy testing using checkerboard based on BMD is more reliable for interpreting the effect of colistin combination.19
Despite no synergistic effects observed in our study, there was no CRAB strain with tigecycline MIC > 2 μg/mL after combination with colistin. Additionally, the CRAB isolates with tigecycline MIC below > 0.25 μg/mL increased up to 83% as susceptible strains. This synergy effect might be use- ful for optimization of tigecycline for CRAB treatment. Thus, we recommend colistin combined with tigecycline to treat CRAB infection as documented therapy when the results from MIC study revealing tigecycline MIC > 0.25 μg/mL
There were several limitations in our study. For example, we included only 12-CRAB isolates, and this study was not intended to study the efficacy of the combination of colistin and tigecycline to prevent antimicrobial resistance. Also, the benefits of colistin-tigecycline combination in real practice need further study.
The results of this study show that there is no synergistic effect between colistin and tigecycline in the inhibition of CRAB. However, the combination of these two drugs is likely to result in a decrease in the MIC of both drugs. A colistin and tigecycline combination should be considered to treat CRAB infection when the pathogen is susceptible to colistin and tigecycline MIC > 0.25 μg/mL Further studies with a larger sample to determine the in vitro synergistic activity of colistin and tigecycline combination is required.
The authors declare that they have no conflicts of interest.