Alprostadil (prostaglandin E1, PGE1) is commonly used for palliative therapy to temporarily maintain the patency of the ductus arteriosus (DA) until corrective or palliative surgery can be performed in neonates with ductus-dependent congenital heart defects such as tricuspid atresia, pulmonary stenosis, pulmonary atresia, interruption of the aortic arch, tetralogy of Fallot, coarctation of the aorta, or TGA with or without other defects. Alprostadil is a synthetic prostaglandin E1 and possesses pharmacological effects, including vasodilation by direct effect on the vascular smooth muscle. DA is constituted by smooth muscle cells which are especially sensitive to alprostadil.1–3 Adverse effects of alprostadil infusion include apnea, hypotension, hypokalemia, bradycardia, gastric outlet obstruction, feeding difficulties, fluid electrolyte imbalance, fever, jitteriness, leukocytosis, cutaneous flushing, and peripheral edema.4–6 There is a lack of cutaneous effects data. Our research presents a neonate who developed migratory polycyclic erythema of urticaria during treatment with alprostadil.
A full-term male neonate was diagnosed with complex heart disease. His diagnosis included Taussig-Bing anomaly, TGA, large subpulmonic VSD, large PDA, severe coarctation, and interrupted aortic arch. The patient was transferred to our institution, a super-tertiary care for probable managementwithin 5 hours after birth. The 3.3 kg infant was delivered by cesarean section. Cyanosis was noted at birth. The hypoxemia and cyanosis did not improve after using an oxygen mask with bag (oxygen saturation 80%). Intubation with subsequent mechanical ventilation was initiated in this case. Ampicillin and cefotaxime were administered for possible early neonatal sepsis. An echocardiogram revealed dextro-Transposition of the great arteries (d-TGA), large subpulmonic VSD, left aortic arch, large PDA, hypoplastic aortic arch, severe coarctation, and interrupted aortic arch. Alprostadil was started within 3.5 hours after birth. Arterial switch operation (ASO) was planned. The patient’s intravenous medications included dopamine, dobutamine, fentanyl, furosemide, calcium gluconate, sodium bicarbonate, potassium chloride, ampicillin, cefotaxime, and heparin.
While alprostadil 0.07 mcg/kg/min was administrated, migratory polycyclic erythema of urticaria was observed over the face and trunk 20.5 hours after alprostadil was started. The migratory nature of this polycyclic erythema developed over minutes or hours. Over the subsequent 8 hours, migratory polycyclic erythema of urticaria was observed over the face, scalp, neck, and head (Figure 1). The urticaria disappeared after alprostadil was decreased from 0.07 mcg/kg/min to 0.06 mcg/kg/min and a concentration of 19 mcg/mL was decreased to 10 mcg/mL for continuous infusion. The patient continued to receive alprostadil and was monitored closely. Alprostadil was subsequently decreased from 0.06 mcg/kg/min to 0.05, 0.04 and 0.02 mcg/kg/min within 11 hours. Migratory polycyclic erythema of urticaria appeared again while alprostadil 0.02 mcg/kg/min was administrated. The multidisciplinary team observed greater benefits in continuing alprostadil than the risks in sustaining oxygen saturation while waiting for cardiothoracic surgery. Alprostadil was then decreased from 0.02 mcg/kg/min to 0.01, 0.005 mcg/kg/min subsequently. As the dose of alprostadil was reduced, the intensity of the urticaria and the area of the body surface involved were diminished. On the third day of life, alprostadil 0.01 mcg/kg/ min was continued; the urticaria resolved and did not recur until the patient was discharged from the hospital and was transferred to a tertiary care hospital for cardiothoracic surgery. No signs of hypotension, bronchospasm, mucous membrane involvement, and anaphylactic shock were observed at any time. The patient’s other intravenous medications were continued. There is no relationship between potential causes of the urticaria and the course of other medications. The dermatology department was asked to evaluate this event, and a diagnosis of acute urticaria from alprostadil was suspected. A skin biopsy was not offered because of immediate transfer to cardiothoracic surgery.
Cutaneous adverse events after alprostadil administration are rarely reported at present, particularly in neonatal cohorts. This research presents the case of a 2-day-old neonate who experienced migratory polycyclic erythema of urticaria associated with alprostadil administration. To our knowledge, there have only been two other case reports7,8 and one published notes and comments9 on this topic. A search of the other literature found two other possible cutaneous adverse events, including neonatal subcutaneous fat necrosis and the harlequin color change association with alprostadil.10,11
Various articles reported it as urticarial. Carter and Garzon7 showed a neonatal urticarial due to alprostadil, and a skin biopsy was compatible with urticaria. Young et al.8 described a rapidly migratory polycyclic eruption in a neonate on extracorporeal membrane oxygenation (ECMO) after receivingintravenous alprostadil, whereas Wheless et al.9 reported a similar case of a 2-month-old boy with complex congenital heart defects on veno-arterial ECMO who developed an unusual migratory polycyclic eruption associated with alprostadil administration.
In the neonatal period, urticarial is rare. Urticaria is an edematous pruritic plaque that is transient in less than 24 hours, and may occur on the skin and mucous membranes. There are many characteristics of urticarial, including annular shapes, serpiginous, forming bizarre polycyclic, or coalesce.12Pathogenesis and etiology involve leakage of fluid into the extravascular space due to increased permeability of small venules and capillaries. The most associated mediator is histamine.12 Urticaria may be allergic or non-allergic. Allergic urticaria related to drug cause mast cell degranulation through IgE receptors on mast cells and basophils by IgE-dependent mechanism such as beta-lactam sensitivity. The amount of drug is not related to the severity of the reaction; small doses may cause severe urticaria. On the other hand, non-allergic urticaria is related to direct release of histamine from mast cell without the need for receptors and IgE-independent mechanisms such as morphine, tubocurarine, and vancomycin.8,12
Theoretically, alprostadil has an effect on histamine release from mast cells or basophil leukocytes in higher concentrations and inhibition of histamine releasing in low concentrations.13 Sondergaard and Greaves14 have also described that alprostadil released histamine in vitro. The cutaneous inflammatory reaction, the wheal, and the erythema were observed. Endogenous histamines were released after receiving intradermal injection of alprostadil. Pretreatment with an antihistamine can reduce the wheal, but not the erythema in this study.14 However, the reaction in our study disappeared due to no antihistamine. Vasodilation causes hyperemia and local edema, in which no signs of edematous involvement may result from cutaneous inflammation of alprostadil.7 The cutaneous adverse events in this case and prior cases8 were related to dosage and amount of alprostadil administration. As suggested, we observed that our patient was more compatible with a non-allergic mechanism, especially since mast cells release direct histamine degranulation and the severity of migratory polycyclic erythema of urticaria varied in correlation with the dose of alprostadil.
Furthermore, this case is not on ECMO, which is a different feature than those discussed by Young et al.8 and Wheless et al.9 Both patients who were or were not on ECMO developed hypoxia. Hence, hypoxia decreased pulmonary blood flow. The metabolism of alprostadil, which is primarily metabolized in the lungs, and an oxygen-dependent process when a patient is or is not on ECMO may relate to the hypothesis in this reaction.8,9,15 So, the exact mechanism of this drug-induced reaction remains largely unknown.
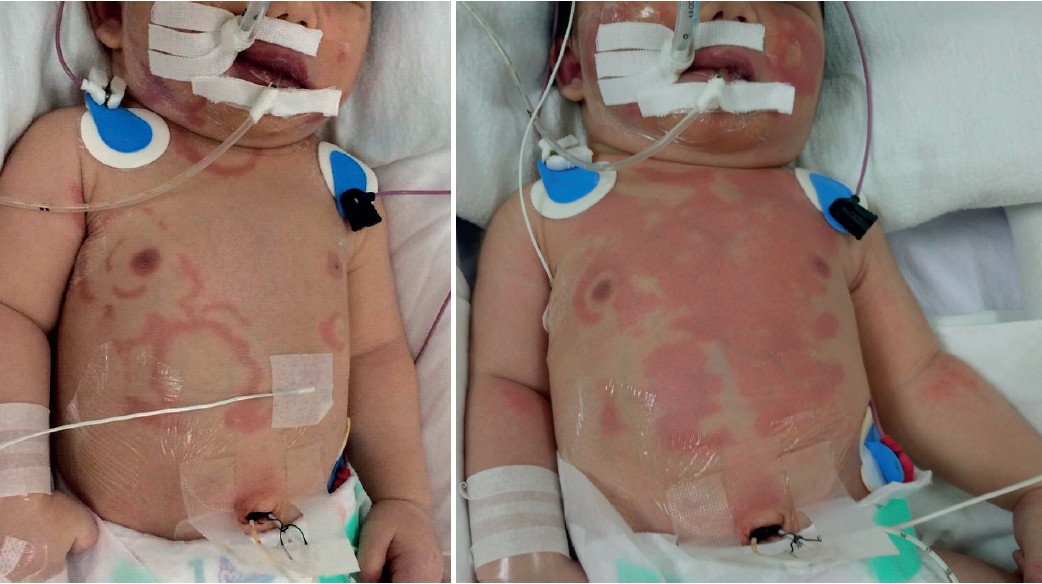
Figure 1: Migratory polycyclic erythema of urticaria associated with alprostadil in a neonate.
We demonstrate a case report of migratory polycyclic erythema of urticaria associated with alprostadil administration in a full-term male neonate. Early diagnosis of this event suggests that discontinuation of alprostadil may not be necessary when alprostadil is a life-sustaining therapy and these adverse drug reactions do not affect the cardiorespiratory system. Treatment may be continued for several days until cardiothoracic surgery can be performed in the absence of evidence of hypotension or anaphylactic shock. There is a lack of systemic reports, and future studies may be needed to under- stand the mechanism of this reaction.
No conflict of interest.
The authors would like to thank the Queen Sirikit National Institute of Child Health’s multidisciplinary team for caring and closely monitoring of the patient’s symptoms, and Ms. Maria Suzanne Mullet for reviewing the manuscript.