Cardiomyopathies are primary (i.e., genetic, mixed, or acquired) or secondary (e.g., infiltrative, toxic, inflammatory) and lead to progressive heart failure with significant morbidity and mortality.1 Dilated cardiomyopathy (DCM) is classified as a mixed (genetic and nongenetic) primary cardiomyopathy. DCM manifests clinically at a wide range of ages and is characterized by ventricular chamber enlargement and systolic dysfunction which leads to progressive heart failure and a decline in left ventricular function, ventricular and supraventricular arrhythmias, conduction system abnormalities, thromboembolism, and sudden or heart failure–related death. Indeed, DCM is a common and largely irreversible form of heart muscle disease. It is the most frequent cause of heart transplantation. Coronary artery disease is one of the most frequent causes of heart failure (HF). There is no uniform definition for ischemic cardiomyopathy (ICM). Despite improvements with medical treatment, the prognosis of patients with end-stage HF remains poor. The median survival was 4 years after the onset of HF. One-year survival was 72% and 5-year survival was 45% after the onset of HF.2 Revascularization of nonviable myocardium has not proven to be beneficial in terms of either mortality or improvement of left ventricular function. Heart transplantation is currently the best treatment option for end-stage cardiomyopathy however it is limited by a shortage of donors. In this study, we only included patients with DCM and ICM.
Stem cell treatment has gained popularity over the past 10 years because it offers a new treatment for end-stage or degenerative diseases such as heart failure, diabetes and spinal infarction. The stem cells used may vary from embryonic stem cell, fetal stem cell to adult stem cell. Embryonic and fetal stem cells are limited to clinical usages due to obvious ethical reasons and the potential for tumor growth in recipients. The induced pluripotent stem cell was newly developed and has great potentials. In cardiology, cardiac resident stem cells are promising. However, stem cells most commonly used in heart failure clinical trials were skeletal myoblasts and hematopoietic stem cells. Skeletal myoblast cells were the frst type used; however skeletal myoblasts injection showed no LVEF improvement compared with controls in the randomized placebo-controlled myoblast autologous grafting in ICM patients (MAGIC trial):3 skeletal myoblast cells were unable to integrate and synchronously contract with the host cardiomyocytes post-implantation.4 The angiogenic cell precursors (ACPs) used in this study were generated from autologous peripheral blood and represent a heterogenic stem/ progenitor cell population of hematopoietic cells that potentially differentiates in vivo in response to tissue signals at injection site and lineage specifc angiogenic precursors.5 Animal experiments demonstrated these cells’ efficacy; a significant reduction of myocardial scarring and increased blood vessel density in the direct intramyocardial injected areas.6 We previously reported the feasibility and safety of using ACPs to treat cardiomyopathy.7
Here we aim to report midterm results and assess clinical markers which can predict outcomes of intramyocardial ACPs injection for cardiomyopathy.
Patient population
Between May 2005 and April 2010, 143 consecutive cardiomyopathy patients gave informed consent and underwent intramyocardial ACPs injection. Sixty patients were dilated cardiomyopathy (DCM) and 83 were ischemic cardiomyopathy (ICM). The study was approved by the Ethics Committee and Institutional Review Board. Screening of severe contagious infections, HIV and hepatitis were done; only patients testing negative were included in the study. Malignancy within the preceding 3 years was also an exclusion criterion. All patients had a recent coronary angiogram (within 6 months) confirming negative coronary artery disease status before the procedure. They all underwent pre-operative workups including routine chest x-ray, electrocardiography, and NT-ProB-type natriuretic peptide (NT-ProBNP). Echocardiography without stressing and/ or cardiac MRI (CMR) was performed in all cases. CMR was conducted using 3.0 Tesla MR Scanner (Achieva 3.0T systems with Philips Quasar Dual gradients, Philips Medical Systems, The Netherlands). Patients were excluded from the CMR study if they had contraindications such as metallic equipment implantation. They all undertook a six-minute walk test, New York Heart Association (NYHA) functional class evaluation and routine laboratory tests required for general anesthesia. Quality of life was evaluated at preoperative and postoperative periods with Short Form-36 (SF-36, a multi-purpose, short-form health survey). The SF-36 consists of 36 items or questions grouped into eight healthrelated aspects of the patient’s life. The scores for each aspect can vary from 0–100; the higher the scores the better the quality of life. The score of 50 was considered normal. Patient’s clinical characteristics are summarized in Table 1.
Table1: The clinical characteristics of patients
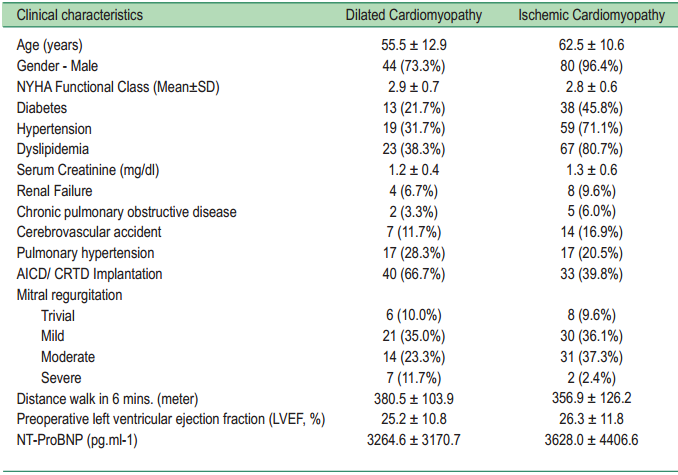
NYHA = New York Heart Association, AICD = automatic implantable cardioverter defbrillator,
CRTD = cardiac resynchronization therapy with defbrillator, NT-ProBNP = NT-ProB-type natriuretic peptide
The cells
The adult stem cells used in this study are “Angiogenic Cell Precursors (ACPs)” developed by VesCell technology (VesCell™, TheraVitae Co. Ltd., Ness Ziona, Israel).5 ACPs are derived from the patient’s own blood, avoiding immunological concerns. Peripheral blood of 250 ml was collected from the patients and sent for cell expansion. Blood cultures for aerobic and anaerobic bacteria were also collected and confirmed negative results. Multipotent progenitor cells were isolated from the blood, rich in CD45, CD31Bright, CD34+CD45-/Dim and CD34Bright cells. The cells at a concentration of 1.5-3.0 x 106 cells/ml were then cultured with vascular endothelial growth factor (VEGF, R&D Systems, Minneapolis, MN, USA) and 5 IU/ml heparin (Kamada, Beit-Kama, Israel). Number and viability of cells were checked and passed quality controls before their use. The product (consisting of at least 1.5 ± 0.5 million autologous endothelial progenitor cells isolated from the patient’s blood and then expanded ex vivo under sterile conditions over 5 days) was suspended in 15 ml sterile cell culture medium. Acceptable culture parameters (assessed by microscopy and flow cytometry) conformed to these specifications: Cell viability ≥ 75% and Morphology –spindle-shaped, large cells forming long thread-like structures.
Sterility tests were performed according to 21 Code Federal Regulation (CFR) 610.12. Assessment of cell culture sterility was performed on a sample of the cell fraction supernatant or phosphate buffered saline (PBS) following cell washing. Interim negative sterility results of all samples taken at different stages of the culture were compulsory for the release of the final product. The Bacterial Endotoxin test was performed according to United States Pharmacopeia (USP) 23. The Limulus Amebocyte Lysate (LAL) test was performed on a sample of supernatant taken from the cell culture. Endotoxin levels below the acceptable limits were compulsory for the release of the final product. Gram stain was to assess bacterial contamination of tissue culture samples. Negative results of the Gram stain performed on samples taken from the washing medium of cells before vialing was compulsory for the release of the final product. Mycoplasma contamination was tested and a negative result of the test was also obtained. The product phenotype was assayed by immune staining, as well as for angiogenic potential (tube formation assay) and cytokine secretion. All cell preparations complied with pre-defined release criteria of safety and potency.
Immune Staining
Cell samples were washed in PBS and resuspended in 100 µl of PBS, stained with specifc fluorochrome-conjugated nti-human antibodies or isotype-matched non-specific controls, and incubated in the dark for 30 minutes on ice. The following antibodies were used for staining: -CD31-PE or CD31-FITC (IQP, Groningen, The Netherlands); anti- CD34- APC (BD Bioscience, Franklin Lakes, NJ, USA), -CD117-APC DakoCytomation, Glostrup, Denmark); anti -CD133-PE, CD144-FITC, -KDR-PE, -Tie-2-PE (R&D Systems, Minneapolis, MN), and anti-vWF–FITC (Chemicon, Temec la, CA). Ac-LDL uptake was measured by incubating the cells with 0.8 µg/ml Ac-LDL (Alexa Fluor488 AcLDL - Invitrogen, Carlsbad, CA, or Ac-LDL-DiI – Biomedical Technologies, Inc., Stoughton, MA) for 15 minutes at 37oC. Non-viable cells were excluded by 7-Amino-Actinomycin D (7-AAD, eBioscience, San Diego, CA, USA).
Cell suspension triplicates of five hundred thousand cells each were stained, assessed by FACS (FACSCalibur, Becton Dickinson), and analyzed by CellQuest Pro software (Becton Dickinson). The percentage of each marker was determined in each test tube and the mean and % Coefficient of Variance (% CV) was calculated for each one. The results were expressed as mean ± Standard Error (SE) of the percentage of stained cells. The number of stained cells was calculated by multiplying the number of harvested cells by the staining percentages obtained using the FACS.
Tube Formation Assay
The angiogenesis potential of the cells was measured by their ability to form three-dimensional tube-like structures according to a widely-used scale using an in vitro angiogenesis assay kit (Chemicon) and scoring under an inverted light microscope (Nikon ECLIPSE TS-100).
Analysis of Cytokine Secretion
Samples of the harvested cells were washed in PBS and resuspended to one million in 1 ml X-vivo15 and grown for 24 hours in 24-well plates. Cytokine secretion in the supernatant was measured as compared to that of the medium only using flow cytometry and the BD™ CBA Human Angiogenesis Kit (Becton Dickinson).
Number of cells prior to injection was 46.2 ± 36.7 million (3.3-200) with 96.9 ± 3.5 % viability in DCM; number of cells prior to injection was 48.1 ± 37.0 million (1.6-165.6) with 96.4 ± 4.0 % viability in ICM. Cells were injected into all areas of the left ventricle in the DCM. For ICM, cells were injected into non-viable myocardium including interventricular septum and hypokinetic segments of the left ventricle.
Surgical Techniques
All DCM had intramyocardial cell injection alone by thoracoscopic technique or microthoracotomy. Most patients underwent microthoracotomy approach due to better exposure, control and shorter operative time. For ICM, 54 (65.1 %) had ACPs injection alone and 29 (34.9%) combined coronary artery bypass grafting (CABG) with ACPs injection.
Microthoracotomy for ACPs Injection
Under general anesthesia with one-lung ventilation, the patient was placed in the right lateral decubitus position. A 10-cm incision was made in the left chest at the 5th intercostal space on the posterior axillary line. The chest cavity was examined and the pericardium was opened longitudinally anterior to the phrenic nerve. Pericardial traction stitches were placed appropriately to assist in reaching all regions of the left ventricular wall.
Cells were injected with the 23-guage butterfly needle with home-made guard. The needle was brought to the heart and then the injections were done manually outside the chest with the extension line. There were 30 sites of injections, 0.5 ml/ injection. Cells were injected into non-viable myocardium predetermined by CMR or myocardial nuclear scan including interventricular septum and hypokinetic segments. After adequate hemostasis the microthoracotomy was closed with small chest drainage left in the 7th intercostal space opening.
Off-Pump coronary artery bypass grafting (OPCAB)
OPCAB approach was carried out. After 1mg/kg of heparin was given, deep pericardial traction stitches were applied to verticalize the heart. The heart was then stabilized with an Octopus III or Octopus IV stabilizer (Medtronic, Inc., Minneapolis, MN 55432) without using any cardiac positioning device. Systemic systolic blood pressure in both groups was kept above 100 mmHg and central venous pressure/ pulmonary artery diastolic pressure in the 20’s to maintain adequate perfusion. All operative maneuvers were routine.
Anastomoses was usually performed to the left anterior descending (LAD) and diagonal arteries first; or to the highest grade or totally obstructed arteries when the LAD system had less severe obstruction. After the measuring was completed the anastomosis was performed in the usual fashion using side to side anastomosis for sequential and end to side for distal end anastomosis with 4-8 interrupted stitches of 7-0 prolene. Intra-coronary shunt was used only in the large dominant right coronary artery or when patient was unstable or exhibited significant, persisting EKG changes after the occlusion. One right ventricular temporary epicardial pacing wire was inserted in these high risk patients. Protamine was given. Normothermia was maintained with sterile warm blanket throughout the procedure. The intramyocardial cell injections were performed after protamine was given.
Patient follow-up and event assessment
Patients were scheduled for follow-up at 1 month, 3 months, 6 months, and 1 year in the first year, then every 6 months after. Patients were followed for an average of 21.6 months (SD 16.8, maximum 68.9) to determine events. A total of 3,094.9 person-months contributed to the study. Deaths were determined by telephone/e-mail survey and hospital records.
Statistics
Statistical analyses were carried out with SPSSTM for Windows version 10.0 (SPSS Inc, Chicago, IL) and STATA for Windows version 11.0 (StataCorp LP,Texas, TX). Continuous variables are expressed as the mean ± SD unless otherwise indicated. The categorical data was reported as proportion. Paired t-test was used to compare the mean difference of NYHA class, LVEF and scores of quality of life between pre and post treatments. Independent sample t-test was used to compare the mean difference of the LVEF between patients who underwent OPCAB plus intramyocardial cell injection and patients who underwent intramyocardial injection alone.
Probability of freedom of event (death) was calculated according to the Kaplan-Meier survival estimates and measured from the date of surgery to the event (death). Differences between pairs of actuarial curves were tested by log-rank test regarding survival estimates. Cox regression analysis was performed on patients’ baseline characteristics (age, gender, diagnosis, diabetes, hypertension, hypercholesterolemia, pulmonary hypertension, renal failure, NYHA class, serum creatinine, preop LVEF, type of operations and number of ACPs) to investigate, and confirm the associations of different variables determined by univariate analysis, also to identify predictors of mortality after cell injection. Multivariate Cox regression analysis was also performed to determine hazard ratios (HRs) and 95% confidence intervals corrected for confounding factors. A p value of less than 0.05 was considered significant.
Early Clinical Outcome
There was no new ventricular arrhythmia during hospital admission (median = 8 days, range 3-198 days). Thirty-day mortality rate was 3.3% (2/60) for DCM and 8.4% (7/83) for ICM.
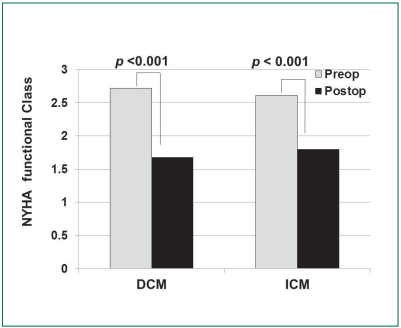
New York Association Functional Class: NYHA class improved from 2.7±0.6 to 1.7±0.7 at 825.1±451.4 days (p < 0.001) in DCM and improved from 2.6±0.7 to 1.8±0.8 at 767.6±549.8 days (p < 0.001) in ICM (Figure 1).
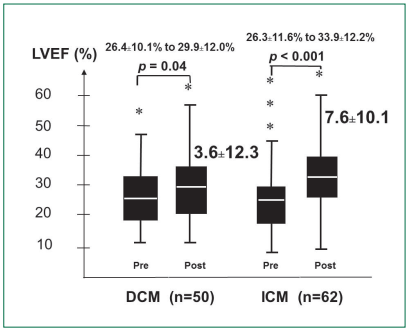
Left Ventricular Ejection Fraction: LVEF improved by 3.6±12.3% (p = 0.04) from 26.4±10.1% to 29.9±12.0% for DCM at 396.3±347.9 days postoperatively. For ICM, LVEF improved by 7.6±10.1% (p < 0.001) from 26.3±11.6% to 33.9±12.2% at 459.8±392.3 days postoperatively (Figure 2). Improvement in LVEF was more in the ICM patients who underwent combined OPCAB plus intramyocardial cell injection than ICM patients who underwent cell injection alone (11.8±11.6% vs 4.9±8.0%, p = 0.007).
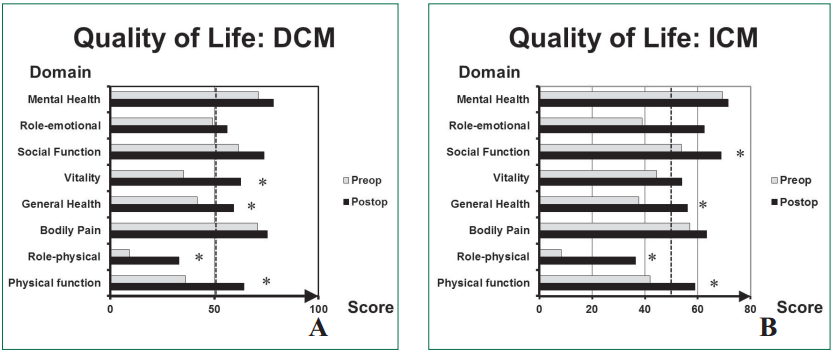
Quality of life: Quality of life postoperatively evaluated at 3 months has significantly improved for physical function, role-physical, general health and vitality domains (p < 0.001, = 0.007,
Survival Analysis
Total follow-up time was 3,094.9 patient-months. Total mortality was 45 of 143 patients (31.5%) during follow-up period, resulting in 14.5 per 1000 patient-months (95%CI: 10.6-19.5) of overall mortality rate. Thirty-six of 132 patients (27.3%) died during follow-up after 30-days. The mortality rate for those surviving the first month was 11.6 per 1000 patient-years (95%CI: 8.2-16.1).
One DCM patients underwent heart transplantation. Overall cardiac death was 43 of 45 patients. Cardiac death included all patients with cardiac-related deaths (28/45, 62.2%) or deaths of unknown causes (17/45, 37.8%). Two patients died of cancer at 599 days and 1,177 days after cell injection.
Overall survival probability at 12, 24, 36 and 48 months after treatments were 79.9% (95%CI: 72.1-85.8), 67.9% (95%CI: 58.5-75.6), 62.9% (95%CI: 52.4-71.7), 55.4% (95%CI: 43.0-66.2), respectively (Figure 4A). Table 2 lists differences in characteristics between survivors and nonsurvivors. Nonsurvivors had higher NYHA functional class and lower LVEF.
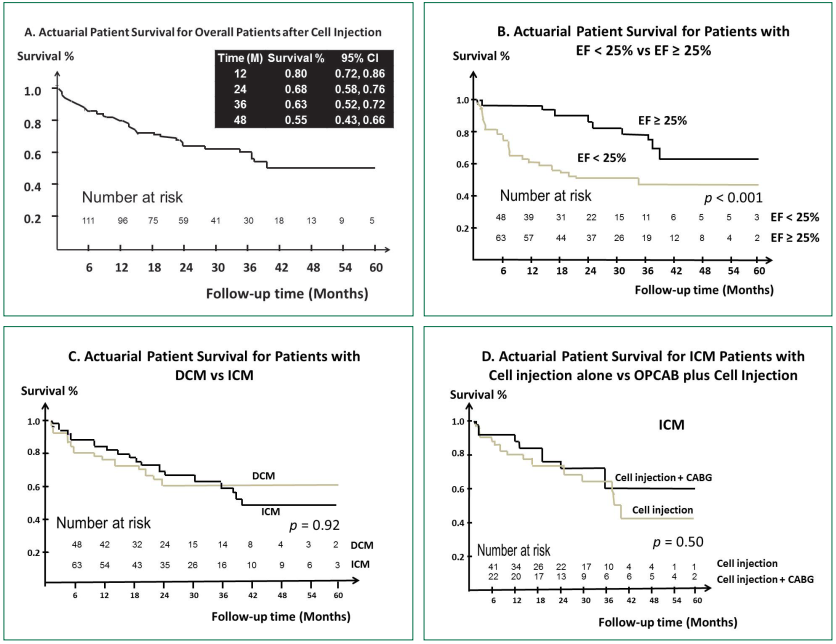
Figure 4: Survival curves for intramyocardial cell injection for cardiomyopathy calculated using the Kaplan-Meier method.
A: Overall survival, B: LVEF less than 25% vs LVEF equal or more than 25%, C: DCM vs ICM, D: Cell injection alone vs OPCAB
plus cell injection.
Table2: Characteristics of survivors and non-survivors
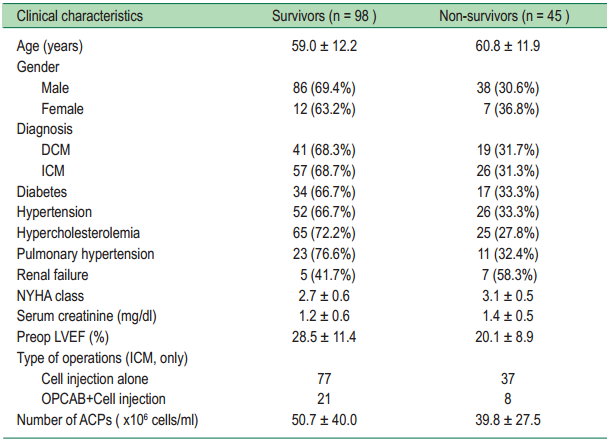
DCM = Dilated Cardiomyopathy, ICM = Ischemic Cardiomyopathy, NYHA = New York Heart Association,
LVEF = Left ventricular function, OPCAB = Off-Pump coronary artery bypass grafting, ACPs = Angiogenic Cell Precursors
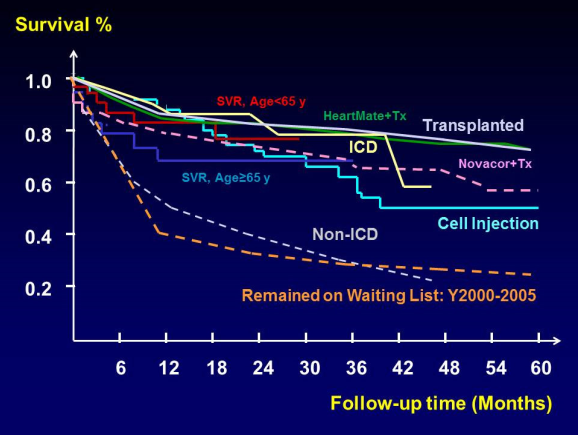
Figure 5: Comparative actuarial patient survival curves between cell injection to studies on other heart failure treatments [including medical treatments, implantable cardioverter defbrillator (ICD) implantation, surgical ventricular restoration (SVR), ventricular assisted devices (HeartMate and Novacor) and heart transplantation (Tx)].
Factors associated all-cause mortality – Kaplan Meier Estimates Survival: For overall patients, the following variables were associated with all-cause mortality after cell injection: age (p = 0.03), renal failure (p = 0.02), NYHA class (p = 0.004) and preop LVEF (p < 0.001, Figure 4B). There was no statistically significant difference of survival after the cell injection between ICM and DCM (p = 0.93, Figure 4C). Subgroup analysis showed hypercholesterolemia (p = 0.02) and preop LVEF (p = 0.003) were significant associated with all-cause mortality in DCM and renal failure (p = 0.02), NYHA class (p = 0.001), preop LVEF (p = 0.008) were significant associated with all-cause mortality in ICM. There was no statistically significant difference of survival after the cell injection between patients who underwent cell injection alone and patient who underwent OPCAB plus cell injection (p = 0.5, Figure 4D).
Risk Predictors for all-cause mortality – Cox proportional hazard model: Cox regression analyses suggested that only preop LVEF (hazard ratio 0.91, 95%CI 0.87-0.95, p < 0.001) was associated with decreased survival.
This study demonstrates the safety of intramyocardial peripheral blood stem cell transplantation in patients with DCM and ICM. The 30-day mortality was about 6%. With increasing clinical experience, mortality could be lowered in both groups. The efficacy of the cell injections was demonstrated, comparing postoperative and preoperative NYHA functional class, LVEF and quality of life. NYHA functional class, LVEF and half of the domains in quality of life questionnaire were significantly improved in both DCM and ICM.
In summary, survival after intramyocardial cell injection was 79.9% after 1 year, 67.9% after 2 years, 62.9% after 3 years, and 55.4% after 4 years (Figure 4A). Only preop LVEF (hazard ratio 0.91, 95%CI 0.87-0.95, p < 0.001) was associated with decreased survival. Patients with LVEF over 25% had a better survival. In comparison to studies on other heart failure treatments, (including medical treatments, Cardiac resynchronization therapy, devices for ventricular reshaping, surgical ventricular restoration, ventricular assisted devices and heart transplantation), survival was slightly better in the early phase about 1 year after cell injection. Survival after cell injection was better than for patients awaiting heart transplantation or those without AICD/ CRTD implantation (Figure 5).8-11
Among available medical therapies, the addition of a β-blocker to conventional therapy was associated with a significant impact on morbidity and mortality. Angiotensin receptor blockers (ARB) are superior to angiotensin-converting enzyme inhibitors (ACEI) in reducing all-cause mortality or hospitalization in treatment of heart failure. Aldosterone blockers effectively reduces mortality in patients treated with an ACEI/ ARB and a beta blocker in the patients with acute myocardial infarction and chronic heart failure.12 Digoxin is beneficial in terms of reduction of hospitalization in patients who had lower LVEF and systolic blood pressure, higher prevalence of males, third heart sound and peripheral edema.13
Except for heart transplantation, all other available treatments mostly treat the end results of the pathological changes in heart failure and do not take care of the causes (cardiomyocytes). The advances made in transplant candidate management, selection, surgical techniques, immunosuppression and post-transplant patient care have resulted in significant improvements in patient outcomes. The mortality rate was 12% at 30 days (95%CI: 11-13%) and 19% at 1 year (95%CI: 17-21%) after heart transplantation. Survival after 5 years was 71% (95%CI: 68-73%) and after 10 years was 56% (95%CI: 53-59%).14 However the shortage of donor hearts remains problematic, especially in elderly ICM patients who may have increased risk for immunosuppression related complications like, renal failure or malignancy.15 However, there is a high risk of death while awaiting transplantation.
Research results on outcomes of stem cell injection for DCM were limited. Vrtovec B., et al.,16 investigated the effects of intracoronary transplantation of CD34+cells in patients with DCM. Twenty eight patients were randomized to have intracoronary peripheral blood CD34+ cells injection which were mobilized by granulocyte-colony stimulating factor and collected via apheresis compared with control. At 1 year, intracoronary peripheral blood CD34+ cells injection was associated with an increase in LVEF (from 25.5 ± 7.5% to 30.1 ± 6.7%; p = 0.03), an increase in 6-minute walk distance and a decrease in NT-proBNP. The 1-year mortality or heart transplantation was lower in patients receiving cell injection (2/28, 7%) than in controls (8/27, 30%) (p = 0.03).
Stem cells for treatment of myocardial infarction and ICM that are under-investigated clinically are mainly hematopoietic stem cells. Bone-marrow or peripheral blood derived stem cells have however shown positive in other clinical trials in both acute and chronic myocardial ischemia/ infarction.17-19 LVEF improvement ranged from 2.5-15 percentage points. There have also been negative studies in terms of LVEF.20 The reasons for the disparity in results may be due to the differences in types of cells, e.g. CD34+, CD 133+ or unselected bone-marrow stem cells, dosage of cells, type of patients (acute or chronic myocardial ischemia) and delivery methods. Largescale human clinical trials are underway in which subpopulations of autologous bone marrow-derived cells are re-administered into myocardial infarction patients. In the REPAIR-AMI trial, parameters such as infarct size and LVEF were modestly, yet significantly improved. Combined end point of death, myocardial infarction and necessity for revascularization, was significantly reduced in the cell group two years after intracoronary administration.21
Akar et al.,22 evaluated the long-term safety and efficacy of autologous bone marrow mononuclear cell implantation in patients with ICM (who have at least one ischemic area that cannot be re-vascularized by conventional methods) compared with case match-controls who underwent incomplete revascularization by CABG. Bone marrow cells were aspirated from the iliac crest, were processed on the COBE Spectra (Gambro BCT, Lakewood, CO, USA). Final bone marrow cell preparation (57.5 ± 8.9 ml) contained a mean of 31.9 ± 4.4 × 106 (range 0.05–80.4 × 106) CD34+ cells. Cells were injected at the ischaemic (viable infarct border zone) and ungraftable area in combination with coronary artery bypass grafting. They found that cell injection improved perfusion and LVEF in the treatment group. However, Kaplan–Meier survival estimation at 5 years, including in-hospital mortality, was 78.9 ± 10%, in the cell group versus 71.1 ± 11% for controls (p = 0.48). Event-free survival (cardiac events) including mortality was also similar between the two groups (29.6 ± 13.1% vs 17.1 ± 14.4%; p = 0.99).
Yousef et al.,23 presented the first 5-year follow-up of intracoronary autologous bone marrow mononuclear cell infusion after acute myocardial infarction. An equal number of myocardial infarction patients who were offered the procedure but declined to be control subjects. An early significant improvement in LVEF 3 months and 1 year was followed at 5 years by greater exercise capacity and lower mortality (1 death vs. 7 deaths) in the treated patients.
In this current study, the reasons we delivered the cells by direct intramyocardial injection are: (1) intramyocardial injection is a simple method, (2) the target area of injection can be seen directly, (3) cell retention after implantation is maximized compared with trans-catheter coronary artery infusion or retrograde coronary venous infusion, and (4) do not effect coronary stents. The advantages of using autologous peripheral blood derived stem cells in our study are as follows as autologous cells (1) create no immunologic concern, (2) are easily harvested via blood donation, (3) have no systemic effect during the blood collection for progenitor cell selection and expansion, (4) the cell populations harvested are not in the early phase of development, thus there are no tumor formation issues, and (5) ability of repeated procedure. However, the disadvantages are: (1) the cells may not be as potent as other embryonic or pluripotent stem cells to repair all the damaged areas, (2) cells had limited self-renewal process, therefore the improvement may not last forever, and (3) patients with blood-borne infections such as hepatitis or patients with chronic immunosuppressant could not be treated.
The proposed mechanisms of intramyocardial ACPs injection are paracrine effect, homing signal and possible transdifferentiation of ACPs to cardiomyocytes. Proving this in clinical trials has been difficult, although it is supported by basic science research.
Although this study was a non-randomized study, it did include all spectrums of the common types of heart failure patients. The data were prospectively collected and followed-up. We do not have control group in this analysis. However, we have reported our case-match studies for both DCM and ICM previously. Those patients who had undergone intramyocardial ACPs injection tended to have improvement in NYHA functional class and LVEF than in the controls.24,25
The future researches areas are many: (1) the explorations of new types of cells, e.g. resident cardiac progenitor cell, umbilical cord blood stem cells, induced pluripotent stem cells or combined stem cells, (2) creation of a receptive cell environment, (3) timing of stem cell therapy, (4) non-invasive in vivo cell tracking, (5) pharmacologic manipulation or combined gene therapy, (6) repeated cell injections and (7) heart tissue engineering.
This is not a randomized study. Some patients came from overseas; therefore they could not come for follow-up echocardiogram or cardiac MRI. However, we were able to obtain echocardiogram, NYHA functional class results and quality of life questionnaires from them. Follow-up LVEF was only corrected in 41.3% of the patients and 73% of them was measured by echocardiogram. Assessment of LVEF was performed locally by investigators without involvement of a central core laboratory. As such, it is possible that for each assessment, a different technique may have been used. It is possible that LVEF estimates were originally reported as ranges from echocardiographic assessment and converted to a single number for the purpose of study entry. However, the status of follow-up patients was obtainable for survival analysis.
Intramyocardial ACPs injection is feasible and safe in both DCM and ICM. The NYHA, quality of life and LVEF had significantly improved in both DCM and ICM. The early results and survival were good however long-term survival was not promising. Preop LVEF was only a significant predictor of mortality. Large-scale placebo-controlled studies are needed to confirm that use of intramyocardial ACPs injection in cardiomyopathy is efficacious.
Disclosures and Freedom of Investigation: The “Angiogenic Cell Precursors (ACPs)” developed by TheraVitae Co. Ltd. The authors do not have any financial relationship with the TheraVitae Co. Ltd. The authors had full control of the study, methods used, outcome measurements, data analysis, and production of the written report.
The authors would like to express their gratitude to the late Dr. Kitipan V. Arom, who originally pioneered this project and developed the technique of intramyocardial cell injection; he is greatly missed by all at Bangkok Heart Hospital since he passed away from cancer in May 2011. The authors also thank Ms. Joanna Attridge for her assistance with English editing.