Juxtarenal aortic aneurysms (JRA) account for approximately 15% ofabdominal aortic aneurysms. 1 By definition, suprarenal aortic crossclampingis required for surgical repair, causing temporary renal arteryocclusion that may lead to postoperative renal dysfunction, in some caserequiring (temporary) hemodialysis.
Standard endovascular aneurysm repair (EVAR) is not an option due toinadequate landing zone for the graft below the renal vessels. Hence fenestratedand branched aortic endografts have been developed to treat high risk patientsunfit for open surgery and anatomically unsuitable for standard EVAR. However,procedures are complex, technically challenging, and time consuming.2,3
A 74-year-old man with a past medical history of hypertension,dyslipidemia, ischemic heart disease, chronic renal insufficiency andperipheral vascular disease, underwent right total knee replacement in March2018. Postoperative kidney ultrasonography was performed due to renalinsufficiency which revealed an abdominal aortic aneurysm. His computedtomography (CT) scan of the thorax, abdomen, and pelvis showed a largepararenal abdominal aortic aneurysm (7.8x6.8 cm) (Figure 1).
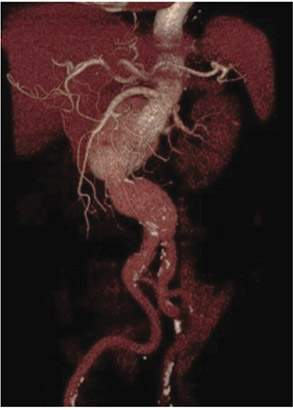
Figure 1: Three-dimensionalreconstruction of computedtomography angiography(CTA) demonstrates a juxtarenalabdominal aortic aneurysm(AAA).
Owing to the significant risk for open surgery, FEVAR wasplanned. The anatomy was suitable for FEVAR but the rightcommon iliac artery (CIA) was dilated, necessitating the extensionof the right iliac limb into the external iliac artery (EIA). Thedecision was made to proceed with coiling of the right internaliliac artery (IIA) one week prior to FEVAR. FEVAR wasperformed in July 2018, 3 months after CTA diagnosis asobtaining a factory customized aortic stent can take 2-3 months.FEVAR is performed in the hybrid or equipped with a flexibleC-arm (Philips Allura Xper FD20, WA, USA). The operationwas performed under general anesthesia via bilateral opencommon femoral artery (CFA) access with the patient undergeneral anesthesia and on heparin therapy (1 bolus dose of 70
The fenestrated stent graft was then fully unsheathed. Thenceliac artery was cannulated with a Terumo 0.035-inch wireover a Simmon1 catheter, which was then exchanged toAmplatz stiff wire followed by a 7F Flexor® Ansel guidingsheath (Cook). BeGraft 8 mm × 27 mm covered stent was thendeployed through the sheath and flared out. Another endograft(Cook® Aortic 28 mm × 80 mm) introduced via the left CFAwas deployed inferiorly as far as the aortic bifurcation andunsheathed. CIA endograft (Cook® iliac limb 13 mm × 107 mm)was deployed (Figure 3). The aortic body was balloon moldedafter each deployment. Final check angiography revealed stentin proper position without any endoleak. All devices and guidewires were removed. Both CFAs were closed primarily using6/0 polypropylene sutures. The patient was extubated andtransferred to the CCU in satisfactory condition. The patientreceived 48 hours of intravenous cefazolin post-operativelyand was monitored clinically for any infection. He wasdischarged well on the fourth post-operative day. Thecomputed tomography angiography at 1 month showed smalltype II endoleak at right posterolateral aspect of aortic bifurcation(Figure 4).IU/kg). With the angiographic aid of a descending thoracicaortic pigtail catheter introduced via left femoral artery access,the fenestrated graft was introduced through the right CFA.The vertical position and rotational anatomy of the fenestrationswere confirmed on angiography. The graft was then partiallydeployed. Using a larger 14F sheath via the left CFA whichwas free, right renal artery, left renal artery and SMA weresequentially cannulated with Terumo 0.035-inch wires overCobra C2 catheters (Cook, Bloomington, IN, USA), whichwere then exchanged for Amplatz stiff wires followed by 7FFlexor® Ansel guiding sheaths (Cook). BeGraft 6 mm × 22mm, 6 mm × 22 mm and 8mm×37mm covered stents were thendeployed through the sheaths and respectively flared out (Figure 2).
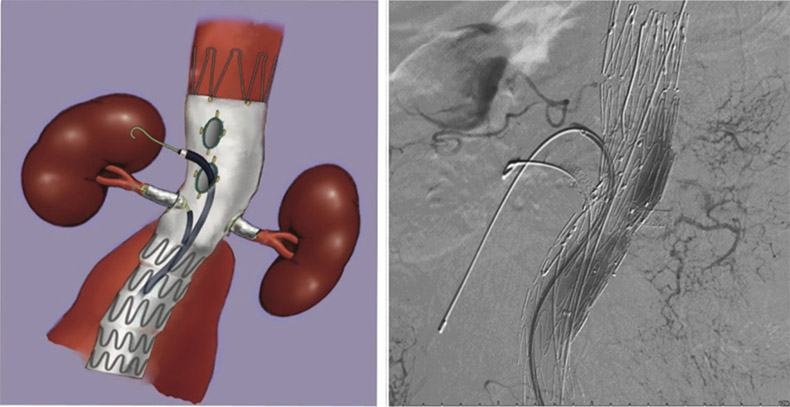
Figure 2: After right renal artery, left renal artery and SMA were sequentially cannulated with Terumo 0.035-inch wiresover Cobra C2 catheters (Cook, Bloomington, IN, USA), which were then exchanged to Amplatz stiff wires followedby 7F Flexor® Ansel guiding sheaths (Cook). BeGraft 6 mm × 22 mm, 6 mm × 22 mm and 8 mm × 37 mm coveredstents were then deployed through the sheaths and respectively flared out.
Open surgery for juxtarenal aortic aneurysm, if comparedwith infrarenal aortic aneurysm, is characterized by moreextensive mobilization of viscera to achieve adequate exposureof the abdominal aorta and by a period of renal ischemia,potentially increasing operative risk, especially the risk ofpostoperative renal dysfunction. This complex aortic pathologyremains a challenge for suitable endovascular repair withoutcompromising visceral perfusion. Even though the advancesin stent-graft technology now permit aneurysm necks ≤ 10 mmto be treated by classical EVAR. However, in standard EVARthe complication rates climb rapidly with increasing numbersof adverse features in the proximal neck.
Fenestrated endovascular aneurysm repair (FEVAR) wasfirst reported in 1999 for the treatment of a juxtarenal aorticaneurysm. The technique was initially developed to treat highrisk patients unfit for open surgery and anatomically unsuitablefor standard EVAR. Not all JRAs are suitable for FEVAR. It is clear that a large number of patients are excluded fromEVAR due to inadequate aneurysm neck length.4 There are asyet no data extrapolating this to identify the proportion ofpatients eligible for FEVAR.
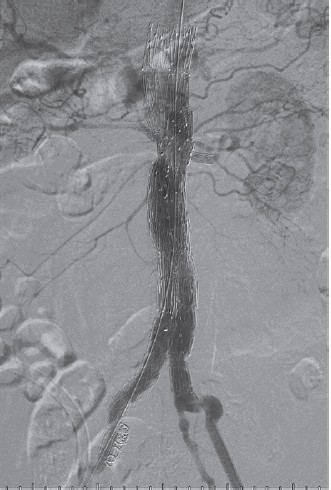
Figure 3: Completion angiographydemonstrates widely patent fenestratedstent graft with no endoleak.
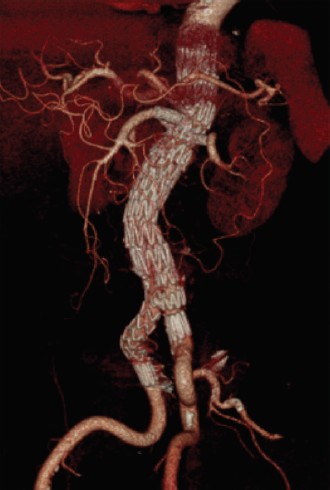
Figure 4: follow-up CTA at 1 monthsshows a widely patent fenestrated stentgraft with small type II endoleak at aorticbifurcation level.
Custom design and manufacture of these grafts excludestheir utility in the acute setting. Current time to manufactureis 6-8 weeks. ‘‘Off-label’’ surgeon-modified devices have beendescribed, on-site modifications of commercially availableaortic endografts.5 More complex procedures may increaseoperating time and rates of graft-related endoleak. Until an‘‘off-the-shelf’’ device is created this remains the only optionin emergencies where open repair is prohibited.
A recent study reported outcomes of FEVAR as a first linestrategy for short neck, juxtarenal, and suprarenal aorticaneurysms in a large volume center, Technical success is96.8%, thirty day mortality is 0.7% and freedom from reinterventionrate for FEVAR at one and 3 years are 96.1% ±1.4% and 90% ± 2.7% respectively.6 This is a consistentfinding in EVAR. Interventions appear to occur within the firstpostoperative year and subsequently plateau. Close surveillanceis essential to identify visceral vessel stenosis or pre-occlusion.Robust statistical comparison of FEVAR versus traditionalopen surgery would only be possible in a prospective randomizedcontrolled trial. No such trial has been devised in the 10 yearsof evolution of FEVAR and it may never happen.
Fenestrated endovascular stent grafting is a feasiblealternative for treatment of complex abdominal aorticaneurysms in high risk patients with a high technical successand low operative mortality and morbidity rates.