Breast cancer is the leading site of new cancer patients in Thai females (41.96% of all female cancers) according to Thai cancer registry of 2015.1 Mammogramphy is widely accepted for screening breast cancer. However, the low sensitivity of mammography for dense breasts, women younger than 50 years of age and small-sized cancers remains a major limitation.2 The use of ultrasonography as adjunctive tool to mammography increases detection rate of screening mammography.3 There is a shortage of both physicians and well-trained technologists to perform breast ultrasound screening.4
Automated breast ultrasound (ABUS) is a novel approach for breast screening ultrasound in which the image acquisition is uncoupled from the interpretation. The study is reviewed by the radiologist on a dedicated workstation using the entire dataset for interpretation. Compared with technologist-performed bilateral handheld ultrasound, in which representative images are presented for interpretation, automated breast ultrasound allows the physician to interpret the entire study and identify the suspicious lesions. Furthermore, automated breast ultrasound allows improved consistency and reproducibility of images, minimizes operator dependence, and aids with inclusion of the entirety of the breast. It does not require physician time for image acquisition and allows review of the study at either the time of acquisition or a later time.
ABUS is also cost-effective.5 Therefore the U.S. Food and Drug Administration approved ABUS as the only approved system of its kind for screening women with dense breast tissue.
Our department started installation of ABUS screening in a bus in order to perform adjunctive breast ultrasonography for mobile breast cancer screening. We present our initial experience and discuss its role in breast screening.
From 1 November 2015 to 31 July 2016, 110 asymptomatic women (age range, 20 - 69 years, average, 39 years) were enrolled to perform automated breast ultrasound (ABUS) to compare results to the standard handheld ultrasonography (HHUS) in our hospital. Among all participants, 79 subjects performed screening mammography and ABUS following by standard HHUS. However, mammograms were not accounted for BI-RADS categorization.
HHUS was set as a standard test performed by 4 radiologists and 3 well-trained ultrasonographers. All radiologists have more than 10-years’ experience in breast imaging interpretation. Whereas ultrasonographers have 1-year experience in breast sonography. However, all positive lesions were approved by experienced radiologists. HHUS machines were Logiq S8 (GEmedical) (Figure 1) and Aixplorer (Supersonic Imagine) (Figure 2) equipped with 50-mm linear arrayed transducers with a bandwidth of 5-12 MHz. For each lesion, we recorded the location according to the breast clock-face position.
For ABUS, we used ACUSON S2000TM (Siemens, Germany) an ultrasound system that automatically acquires full-field volumes of the breast (Figure 3). The system is equipped with an ultra-wide linear transducer (Siemens 14L5BV, 14 MHz, 15.4cm, and 768 piezoelectric elements). This system is installed in our specially designed bus used in mobile screening services. Standard coronal, sagittal, and axial views were taken of each breast. Obtained volume data were migrated to a workstation for multi-planar reconstruction and image analysis.
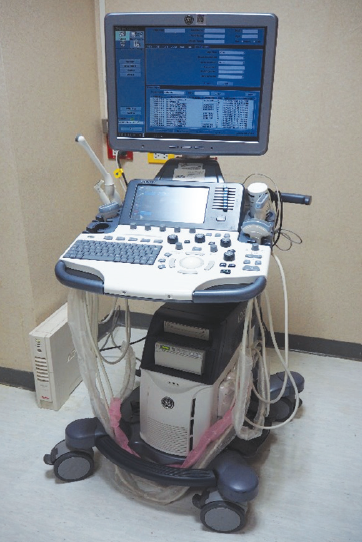
Figure 1: The Logiq S8 (GE medica] ultrasound machine used for handheld ultrasnography of breast
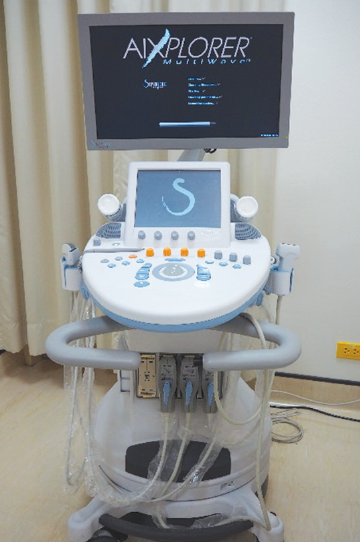
Figure 2: Aixplorer (by Supersonic Imagine) used for performing handheld ultrasonography
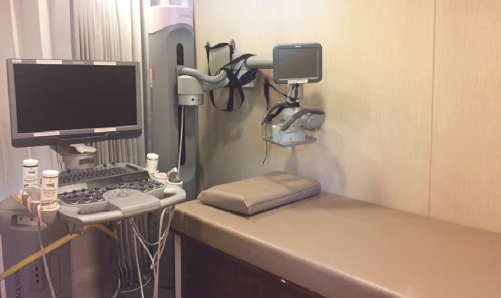
Figure 3: Acuson S2000 Automated Breast Volumertric Scanner (ABVS) By siemens healthcare.
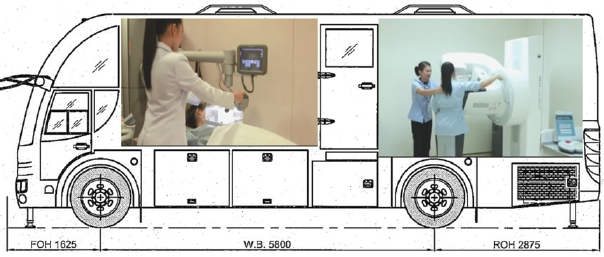
Figure 4: Figure shows installion of ABVS at the front and mammography equipment at the back of a bus.

Figure 5: The side view of breast cancer screening bus including both ABVS and mammography equipments
To compare the position of lesions, we defined concordant position if lesion locations for ABVS and HHUS were not differing by greater than half an hour clock-face location. Four radiologists evaluated the 3D volume data at the automated breast ultrasound work station (Figure 6). They took part in a 3-day tutorial explaining the operation of the ABUS review workstation. The radiologists were blinded to clinical information, the corresponding mammographic findings and the HHUS findings.
Lesions were categorized according to the Breast Imaging Reporting and Data System criteria of the American College of Radiology (ACR BI-RADS®-US)6 as follows:
BI-RADS 1: negative
BI-RADS 2: benign
BI-RADS 3: probably benign
BI-RADS 4: suspicious of malignancy BI-RADS 5: highly suggestive of malignancy
We limited each participant to having only the highest final BI-RADS category. For subjects who have multiple lesions, the highest BI-RADS lesion was selected for comparison with standard HHUS. In these situations, we first separately finalized BI-RADS category before subsequently comparing concordant location of lesion to prevent bias.
For BI-RADS 4, we did not subcategorize lesions to BI-RADS4a, 4b and 4c, because in our daily work flow, recommendation for all subcategories of BI-RADS-4 lesion should be biopsied. Patients with a history of breast surgery, bra cup size greater than size D and inflammatory condition of the breast were excluded.
When comparing the concordance of ABVS with the reference standard (conventional HHUS), the Cohen’s Kappa value as an estimation of the inter-rater reliability was used.7 A kappa value of 1.0 denoted perfect agreement; 0.81–0.99, almost perfect agreement; 0.61–0.80, substantial agreement; 0.41–0.60, moderate agreement; 0.21–0.40, fair agreement; and 0.20 or less, slight agreement.

Figure 6: ABVS workstation, a computer unit with installation of specific software for reviewing ABVS image dataset
We compared the results of the BI-RADS®-US classification by ABVS data with the results of HHUS, (which can be seen as the gold standard) as shown in Table 1.
With respect to agreement cases between HHUS and ABVS, a total 102 of 110 cases (92.7%) agreed. 51 cases (46.36 %) were BI-RADS 1. Twenty-five cases (22.73 %) were BI-RADS 2. Twenty four cases (21.82 %) were BI-RADS 3. Two cases (1.82 %) were BI-RADS 4. No BI-RADS 5 case was detected on both examinations.
Cohen’s Kappa value as an estimation of the inter-rater reliability regarding this comparison was κ = 0.885, reaching an agreement that can be described as almost perfect between both modalities.
Table 1: Agreement between reference standard and experimental ABVS data interpretation focusing on the correct finalization according to ACR BIRADS-US system.
Diagnostic performance in the subgroup of BI-RADS-HHUS 1 breasts
We analyzed the dataset of BI-RADS –US 1 breasts, which exhibited no verifiable lesion (n= 55); 51 (92.7%) were correctly described as BI-RADS US 1. In one case, ABVS revealed two tiny cysts (less than 5 mm in diameters) in R6 and L1 clock-face locations. There are 3 cases of HHUS BI-RADS –US 1 that ABVS described as BI-RADS –US 3. Among these cases, all ABVS BI-RADS –US 3 lesions are oval-shaped hypoechoic lesions with circumscribed margins and orientating parallel with skin surfaces (less than 1.0 cm in diameters). No category BI-RADS – US 4 or 5 was detected.
Diagnostic performance in the subgroup of BI-RADS-HHUS 2 breasts.
Twenty-five cases of 27 HHUS BI-RADS –US 2 were correctly finalized by ABVS (92.6%). These lesions are cysts and clusters of micro-cysts. There were no HHUS-BI-RADS 2/ ABVS –BI-RADS 3, 4 and 5.
Diagnostic performance in the subgroup of BI-RADS –HHUS 3 breasts.
Of 26 cases of HHUS-BI-RADS 3, there are 24 cases (92.3%) that ABVS correctly categorized lesions. An example of agreed BI-RADS-US 3 is shown in Figure 7A and 7B. One case of HHUS-BI-RADS-US-3/ ABVS BI-RADS-US-1 was a tiny cyst (less than 4 mm in diameter in R3 clock face location.) For a case of HHUS-BI-RADS-US 3/ ABVS BI-RADS –US -2, HHUS showed a 9.5x9.2x6.1 mm oval-shaped hypoechoic lesion in the R3 clock-face position whereas ABVS described as a 9.0x7.5x6.5 mm simple cyst.
Diagnostic performance in the subgroup of BI-RADS –HHUS 4 breasts.
There are 2 cases that ABVS correctly finalized as BI-RADS-US 4. The first case is a 41-year asymptomatic woman, whose both ABVS and HHUS depicted a round hypoechoic mass with indistinct microlobulated margins, orientating not-parallel with skin surface (Figure 8A, 8B). No posterior acoustic feature was found. HHUS measured lesion size was 9.3x7.4x10.4 mm whereas ABVS measured lesion size as 8.6x8.4x10.0 mm. This woman was not biopsied by our institute. However, a follow-up interview with the patient noted this mass was a malignancy and the patient underwent a mastectomy in another hospital.
The second BI-RADS –US 4 case is a 39-year old asymptomatic woman. She has an R10 hypoechoic lesion with indistinct margins and posterior acoustic shadowing is detected (Figure 9A, 9B). The lesion is quite small (3.7x3.8x5.0 mm in size). Ultrasound guided core needle biopsy was done. Histology reported usual ductal hyperplasia without malignancy. The patient did not want excisional biopsy, therefore the breast surgeon made an appointment for follow-up ultrasonography.
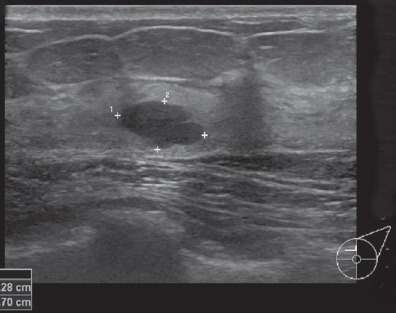
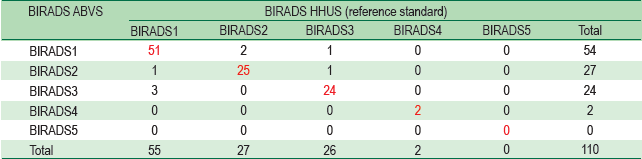
Figure 7A: Handheld Ultrasound Image shows a BI-RADS-US 3 lesion (block arrow) located about L11 clock-face position. It is quite an oval shape, with circumscribed margins, orientating parallel with skin surface and without posterior acoustic features.
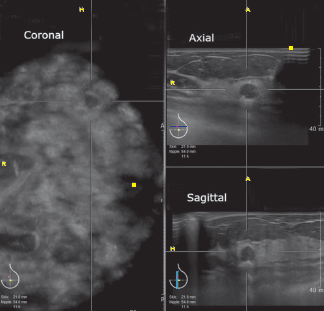
Figure 7B: ABVS images of the same subject of Figure 7A of which mass was finalized as BI-RADS-US 3 concordant with HHUS image.
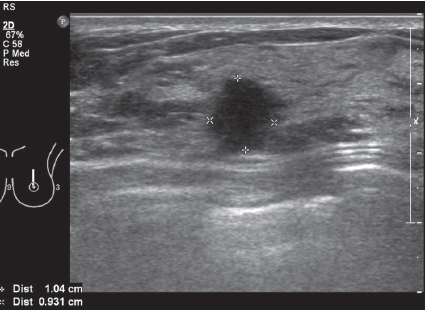
Figure 8A: HHUS-BI-RADS 4 lesion: the mass is quite a round shape, microlobulated margins, orientating perpendicular to skin surface.
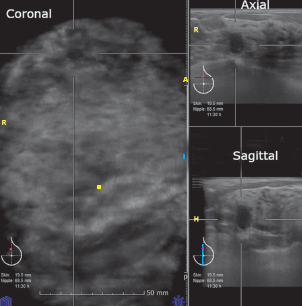
Figure 8B: ABVS images of BI-RADS-US 4 lesion (the same patient as Figure 8A). This mass located about 11:30 clock-face position of the left breast. It has somewhat spiculated margins, orientated perpendicular to skin surface.
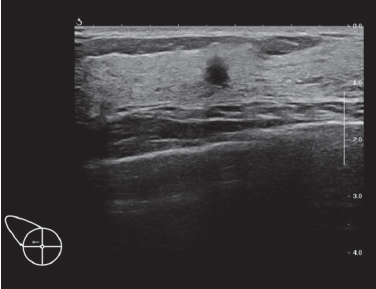
Figure 9A: HHUS image showing BI-RADS 4 lesion: HHUS shows a small hypoechoic lesion which is quite round in shape and orients perpendicularly to skin surface. It has ill-defined margins.
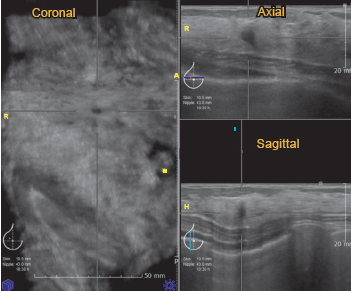
Figure 9A: HHUS image showing BI-RADS 4 lesion: HHUS shows a small hypoechoic lesion which is quite round in shape and orients perpendicularly to skin surface. It has ill-defined margins.
Diagnostic performance in the subgroup of BI-RADS –HHUS 5 breasts.
No BI-RADS-US 5 case was observed in this study
Several studies have suggested that ABUS will play a promising role in the screening of women with dense breasts requiring mammography. Kelly et al.8 performed one multicenter prospective study comparing a combination of screening mammography and semiautomated whole breast ultrasound (WBUS) with the individual methods alone in 4,419 women with dense breast tissue and/ or at increased risk of breast cancer. The results of this study were promising, with an additional 3.6 cancers detected per 1,000 women screened. The sensitivity of the semiautomated WBUS alone was 67% (38/57) and that of mammography alone was 40% (23/57); however, the sensitivity of the combined modality was 81% (46/57) Kelly et al.8 also evaluated the radiologist reader performance for cancer detection in women with dense breasts by using ABUS. They reported that the radiologists were able to improve cancer detection, with an increase of 63% in the callbacks of cancer cases and only a 4% decrease in the correct identification of true-negative cases.
Golatta et al.9 prospectively assessed a large number of 983 patients with 1,966 breasts. They reported a high negative predictive value of 98% (1,520/1,551), a high specificity of 85% (1,520/1,794), and a sensitivity of 74% (88/119), based on the cases of US-guided biopsy. Therefore, they suggested that ABUS might be a promising tool in breast imaging, particularly in screening settings.
We conducted this study to verify and recommend to our institute the policy to implement ABUS as a substitute to HHUS. ABUS has several advantages, compared to HHUS.10
First, ABVS is consistent and reproducible, while HHUS is operator dependent, meaning that the ability to detect and accurately document clinically significant findings in the case of HHUS is dependent upon the experience and expertise of the person performing the scanning.11 Our study agrees with this ABVS advantage in cases of HHUS-BIRADS-1/ ABVS-BIRADS 2 and 3. If we want to check whether HHUS is true negative, it may need a second-look HHUS examination. On the other hand, in cases of HHUS-BIRADS-2 and 3/ ABVS-BIRADS 1, we subsequently reviewed ABVS in the work station setting again and found all small BI-RADS 2 and 3 lesions that we had missed with no need to call the patient back to repeat ABVS.
Second, anyone can be trained to operate the ABUS equipment for scanning, while HHUS has to be conducted by a US technologist or a physician with the knowledge of US physics and anatomy. In our institute, ABVS was performed by trained medical x-ray technologists and public health officers who gradually have more experience over time.
Third, the acquisition time of ABUS is more consistent. The total acquisition time averages 15 minutes for a patient with average-sized breasts and is slightly longer if more than the standard three views of each breast are needed in women with larger breasts. This consistent acquisition time can allocate an appropriate time slot for each patient, without unexpected delays, thus allowing a streamlined workflow. The physician time required by ABUS includes only the time required for interpretation, while the physician time required by HHUS includes the time required for performing the examination and that required for interpreting the results. When a radiologist becomes comfortable with reviewing and interpreting ABUS images, he/she would take approximately 3 minutes to read a negative examination. The time would increase to approximately 5 minutes when there are one or two significant findings. Occasionally, a case may still require 10 minutes or longer.
Our ABVS unit had been installed in a bus for mobile screening breast. This bus prepared two rooms for mammography and ABVS respectively. Thus we could complete work-flow of breast cancer screening out of hospital without radiologists. The dataset was subsequently reviewed at a hospital work station. According to our study, comparison of ABVS to the standard HHUS found an estimation of the inter-rater reliability kappa value as high as 0.886 representing almost perfect reliability. This will support the utility of ABVS installed in a bus for mobile screening breast cancer. This will produce more convenience for patients especially those who reside far from our hospital.
One limitation of ABVS is inadequate scanning of axillary region where breast or solitary malignant lymph node can be detected. Our solution is training our technicians who handle ABVS to perform additional handheld ultrasonography of axillary region in case of suspicious malignant lesion in breast being detected.
The other limitations of ABVS are nipple, small focal shadowing and architectural distortion. If abnormalities are suspicious for malignancy, the patient needs to perform targeted handheld ultrasonography to confirm the lesion.
Our study did not find any BI-RADS 5 lesion. This was probably due to small sample size (only 110 subjects). We will further collect and report breast cancer detection rates in the future as we achieve a larger number of screening cases.
We compared Automated Breast Volumetric Scanner (ABVS) with Handheld Ultrasonography (HHUS) in a screening situation. The study revealed high kappa value (K= 0.885) representing almost perfect agreement based on BI-RADS-US score. Therefore we conclude that ABVS is comparable with HHUS and can be an adjunctive tool to mammography for breast cancer screening.