Aburn wound is a type of skin injury caused by tissue damage after exposure to heat fire, flame or scalds, chemicals, electrical, or radiation.1 Burn causes an estimated 300,000 deaths yearly worldwide and may occur in any age group or sex and in both developing and developed countries.2 If healthcare providers understand the pathophysiology of burn injury, evaluation levels of burn, early resuscitation and good management, this can help to decrease the incidence of burn mortality and mobility. This review presents the pathophysiology of burn injury, and provides an update on current management of burn wound care.
A wound is an injury to a part of the body, especially one in which a break is made in the skin or tissue. There are various types of wounds, including an incised wound, a lacerated wound, abrasions, contusions, ulcers, and burn wounds.3
Wound-healing process Wound healing is the process of healing after physical tissue injury and this process involves soluble mediators such as cytokine or growth factor, blood cells such as platelets and white blood cells, extracellular matrix, and parenchymal cells. Wound healing has four phases: hemostasis, inflammation, tissue proliferation and tissue maturation or remodeling that overlap in time (Figure 1).4,5
1. Hemostasis phase
Skin injury can cause vasoconstriction, which occurs immediately in order to stop the bleeding. Afterwards, platelet aggregation will emerge, accompanied with the release of some clotting factors from the cells which stimulate the coagulation and complement cascades through intrinsic and extrinsic coagulation pathways. Then, prothrombin is triggered to be assembled as thrombin as with fibrinogen, which is transformed to fibrin to stop the bleeding at the wound site. It is not only the platelet release clotting factor that is involved here; they also provide cytokine and growth factors such as Platelet-Derived Growth factor (PDGF), Epidermal Growth Factor (EGF), Transforming Growth Factor-beta (TGF-β), Insulin-like growth factor-1, and platelet factor-IV, which facilitate the wound-healing process.6
2. Inflammatory phase
This phase occurs within 24 hours after the skin is injured and continues for several days or weeks depending on the level of the injury. After blocking the bleeding wound, the aggregated platelets will secrete a chemo-attractant to activate the inflamed cells in order to start the wound-healing process. Specific enzymes are secreted from the cell membrane to stimulate prostaglandins and leukotrienes synthesis. Afterwards, the wound-surrounding tissues and capillary endothelial cells will secrete the histamines which permeate the capillary wall and increase the interstitial space, resulting in fluid flows from the capillaries to the interstitial space. This process causes edema, which has 2 phases: in the initial phase, edema will occur immediately after the secretion of histamine and leukotrienes from mast cell which cause vasodilation. Clotting products such as kinins and thrombin also enhance capillary permeability.
The second phase is caused by the penetration of the fluid through the capillaries. Moreover, there is activation of the mediator combined with peptides called cytokine, which function as cellular immune response enhancers, responding to the antigen and then white blood cells such as macrophage, neutrophil and monocytes are leaked and driven into the interstitial space surrounding the wound. Neutrophils will drive away all of the dead cells by the use of protease enzymes, which degrade the white blood cells. The monocytes are transformed to macrophage, which defends against dead cells and bacteria. After macrophage is released, several types of growth factors, which are angiogenesis factors, stimulate the endothelial cells of wound surrounding capillaries resulting in the generation of new capillaries to the wound, and these also produce some growth factors such as PDFG, EGF and TGF-β which promote the synthesizing of fibroblasts which then move to the center of the wound for proliferation.6,7
3. Proliferation phase
This phase occurs from 3 days up to 2-4 weeks after the injury and might overlap with the end of the inflammatory phase. There is the movement of the fibroblast, which results in the generation of tissues to fill in the injured skin.6 Fibroblast functions as a collagen producer, which causes the wound to adhere. Vitamin C, oxygen and iron are essential for the production of collagen, which is processed in the fibroblast cells through the synthesis of tripeptides.
Neovascularization: After the inflammation phase, local factors in the wound at a microenviromental level such as decrease of oxygen tension, decrease of pH and increase of lactate actually imitate the release of some growth factors to contribute new capillary.8 This process is called neovascularization or angiogenesis and hypoxia induced macrophages secreted angiogenic growth factors such as Vascular Endothelium Growth Factor (VEGF), basic Fibroblast Growth Factor (bFGF), and TGF-β.9,10
Re-epithelialization: The process of epithelialization is stimulated by the presence of EGF and TGF-β, which are produced by being activated at the wound site by macrophage, platelets, and keratinocytes.11 The keratinocytes migrate to cover on the skin surface, and are proliferated and migrate across the wound. When the keratinocytes cover the wound completely with new epidermal cells, the wound is closed completely.
4. Maturation or remodeling phase
The maturation or remodeling phase takes place after the wound is healed. At the beginning, there is redness, swelling, and itchiness. Protein degradation of the unused protein and also collagen rearrangement are processed here. Actually, this phase takes around 6 months but in some cases it could continue for up to 2 years.
All of the wound-healing processes could be summarized as follows: there are natural repairing processes which are complicated, continue, and overlap in order to complete the wound-healing process. For chronic wounds, these processes cannot continue consecutively; the process would be arrested at some phase. Chronic wounds are caused by repeated trauma, ischemia, necrotic tissue, infection, or a disease such as diabetes, including defective white blood cell activity, the arrest of all cells transformation processes, a decrease of fibroblast level resulting in collagen synthesis deficiency, when the growth factor is broken down and which blocks the proliferation phase.
Type of wound healing6
Pathophysiology of thermal burns
1. Local response
The skin functions as the protective barrier which protects the body from the outer environment, preventing dehydration, and controls the body temperature. The extent of the injuries resulting from thermal damage depends on the temperature and exposure period. When the temperature rises to 45˚C, protein coagulation occurs. Douglas Jackson has classified thermal injury into 3 zones. The first zone is called the “zone of coagulation,” which is permanently damaged; the second is the “zone of stasis,” which can change to the “zone of coagulation” if the blood cannot flow through the cells caused by receiving more or less fluids. The last is the “zone of hyperemia.” A burn in this zone can return to normal skin. Thus, the aim of healing is to prevent a change from the zone of stasis to the zone of coagulation.
Within the zone of stasis, there are several inflammatory mediators and vasoactive mediators, such as prostaglandins, histamine, and bradykinin. In addition, an increase in blood vessel permeability can cause wound edema. Moreover, oxygen free radicals, for example, xanthine oxidase, can cause wound edema (Figure 2, and 3).14
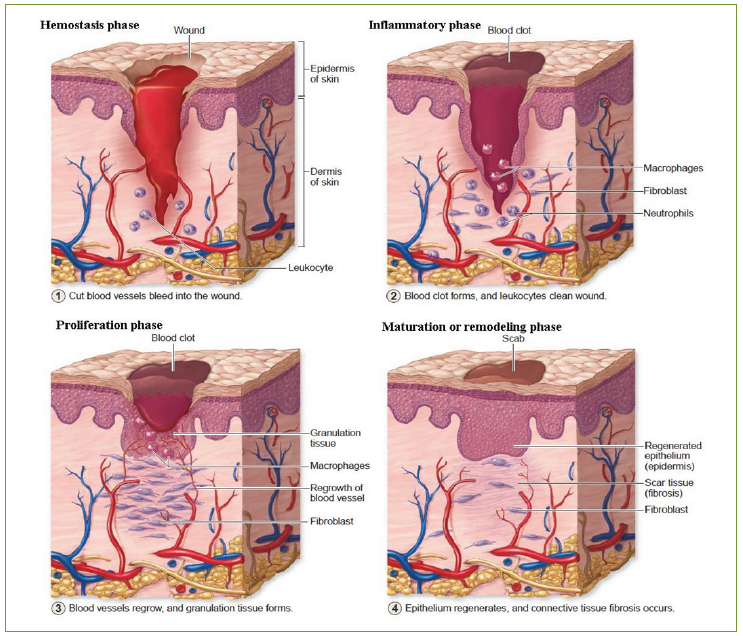
Figure 1: Four phases of wound healing: hemostasis, inflammation, proliferation, and maturation or remodeling phase
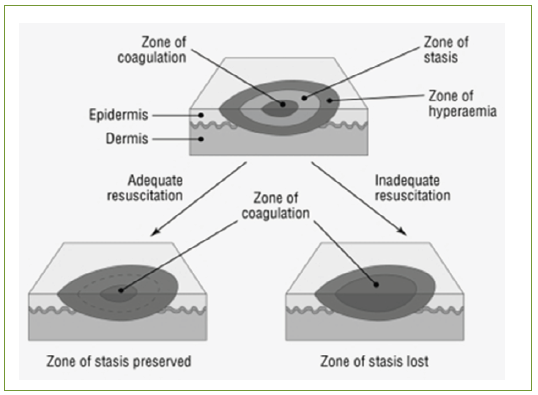
Figure 2: Jackson’s burn zones and the effects of adequate and inadequate resuscitation.14
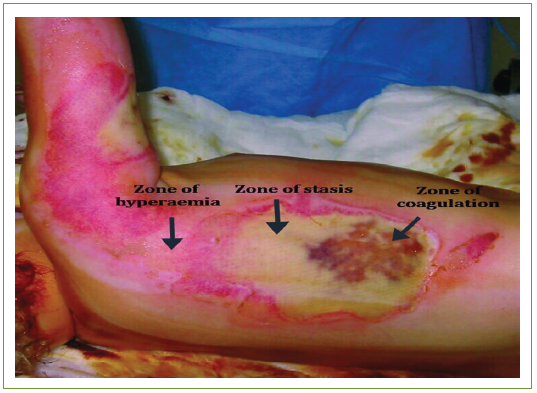
Figure 3: Clinical image of burn zones. There is central necrosis (zone of coagulation), surrounded by the zones of stasis and of hyperaemia.14
Systemic response
Over 30% of burns have a systemic inflammatory response to several cytokines and mediators, resulting in dehydration and hypovolemic, which can affect the oxygen transferred to the cells and subsequently can cause the arrest of the blood flow and a decrease in the heart muscle pressing rate from the tumor necrotic factor. In the case of severe burns, there is a decreased in pulmonary function without inhalation injury which comes from bronchoconstriction caused by histamine, serotonin, thromboxane A2, and also from the decreased lung and chest function (Figure 4).14
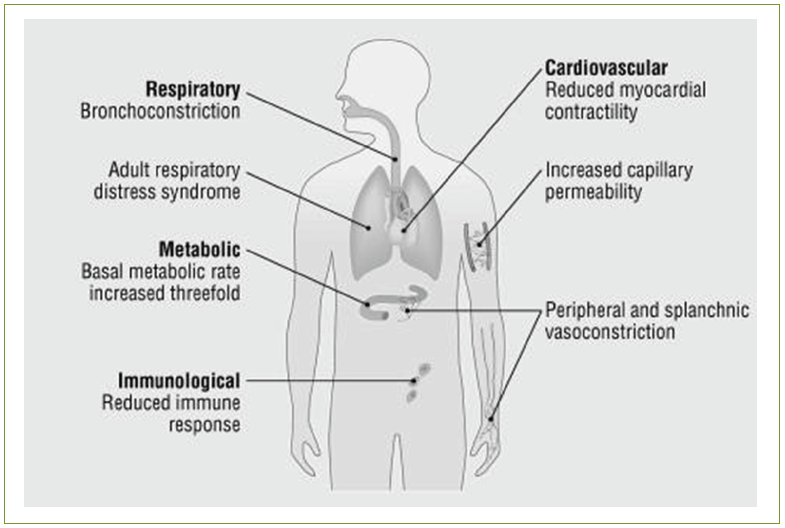
Figure 4: Systemic changes that occur after burn injury.14
A study found that the syndrome was composed of the decreased in mesenteric blood, mucosal integrity and capillary leak, which can allow bacteria to enter into portal circulation and then cause infection in the blood, lungs, skin, and urinary tract. This group of syndromes could be prevented by feeding enough fluid, nutrients and glutamine via blood vessels to promote enterocyte synthesis for curing mucosal integrity.
Regarding the immunological system, it has been found that neutrophil chemotaxis, phagocytosis and intercellular bacterial killing function, decrease in line with cellular-mediated immunity and also lymphocyte activation suppressive mediators.12
Assessment of severity of burn wound
The degree of a burn is considered from the depth of the damaged tissues, which depends on the temperature and prolonged exposure period, divided into 3 classifications (Figure 5).13-15
Classification of burn injury depth
First-degree burns or superficial burns
Superficial burns are not severe; there is partially-damaged skin only in the epidermis layer. The wound is dry and has not erupted but there might be some swelling and a bit of pain, and the surrounding skin will be red. This type of wound can heal automatically within 7-14 days without any scar. Most patients with this type of would can carry on with their daily life as usual without staying in the hospital. Superficial burns are caused by sunlight, steam, flames, and so on.
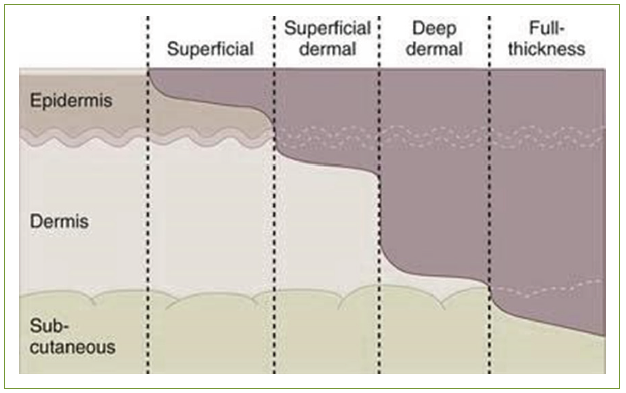
Figure 5: Diagram of the different three-degree deep burn wounds.13
Second-degree burns or partial-thickness burns
Superficial second-degree burns or superficial partialthickness burns partially damage the epidermis and some parts of the dermis layers. The wound is red or dark pink and also has some blisters, swelling, and causes pain. Exposure to hot metal or flaming fluid and also diluted chemicals can cause seconddegree burns, but they can heal themselves in 14 to 21 days.
For deep second-degree burns or deep dermal partial, there is more damage deep in to the dermis layer but the pores and sweat glands are not damaged. The wound is a dry, pale color, and there is the presence of partial blisters, serum leakage, and severe pain. The causes of injury are as previously mentioned. Actually, it typically takes over 21 days to get better and there is always scarring and tissue contraction. This type of wound can be cured by medication or skin graft transplantation or a combination of these methods.
Third-degree burns or full-thickness burns
Here, all of the epidermis and dermis, including the pores and sweat glands, are completely damaged. Moreover, the degree of damage might be deep into the fat layer, fascia, muscle, and bone. The wound is dry, yellow or brown to dark colors. There might be some dark-burnt crust present that is thick and hard textured. Patients will not feel any pain or just a bit of pain due to the nerve ends and nerve nets being damaged. A third-degree burn is caused by exposure to high heat or high voltage electric current or highly-concentrated chemicals for a long period. Furthermore, this type of burn cannot be healed by the human system itself, and therefore surgery or skin transplantation (skin graft) is essential and post burning may be a complication of scar contraction.13
Assessment of the severity area of burn wounds
To determine the area damaged, the total body surface area (TBSA) is defined as 100%, followed by 3 determination methods:13,14
Palmer surface area
Using the patient’s hand with closed fingers determined as 0.8% of the total body surface area is the easiest method to measure a small burn wound (< 15% of total surface area) or very large burns (> 85%, when unburnt skin is counted).
William’s rule of nines
This method is used for adult patients by dividing all of the skin area into a small part which is determined as 9% of the total body surface area as listed in the following:
The advantages of using this method are simplicity and accuracy, but it cannot be used with children because their total body surface area are quite different from adults and they also depend on age (Figure 6).13
Lund and Browder method
The Lund and Browder method is more reliable and can be compared with the previous-described methods. It uses a grid to display the size of the wound, which depends on the patient’s age. This method is usually used for infants to 15 year old children because infants have a bigger head compared with their legs and they will be longer when they grow up (Figure 7).13
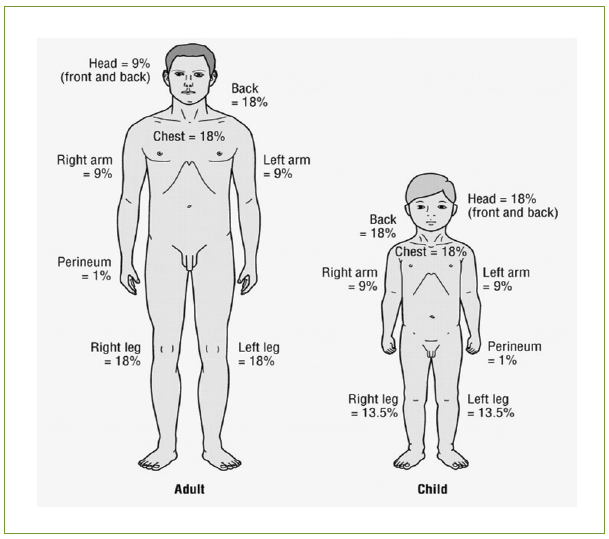
Figure 6: William’s rule of nines.13
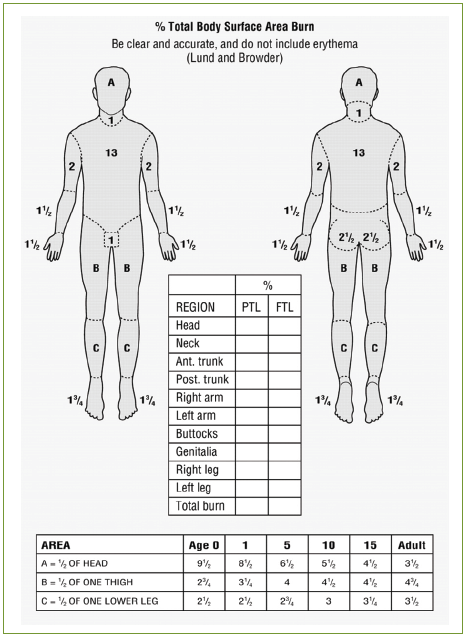
Figure 7: Lund and Browder chart13
Assessment of severity grade of burn wounds
The classification of severity grade according to the American Burn Association (ABA) is divided into 3 grades as follows. 4
Mild or minor burns
This category is for burn wounds which have a damaged area of less than 15% in adults, less than 5% in children, less than 2% in the elderly for third-degree burns, or less than 2% of total body surface area with second-degree burns. This estimate does not include burn wounds on the face or eyes and ears, or reproductive organs, which are classified as first-degree burns. Patients can recover as outpatients.
Moderate burns
In this category, the skin has to be damaged 10-20% in adults, 5-10% in children or the elderly, 2-5% for third-degree burns or 15-25 % of total body surface area with second-degree burn injuries. Patients have to get specific treatment from a specialist.
Severe or major burns
Over 20% of adults, less than 10% of children and the elderly, less than 5% for third degree burns or 25% of total body surface area damaged by second degree burns, including burn wounds on the chest surface, face, ears, hands, feet and reproductive organs, are classified in this grade. The cause of severe injury here might be from high voltage electric current and also inhalation injury. Patients should have specific treatment and observation from expert physicians.
Current management of burn injury16,17
The goals of burn treatment could be survival of the patient with rapid healing of the wound, with minimal scarring and abnormal pigmentation. The most important treatment of a burn wound is to reduce the morbidity and mortality and the prevention of organ function damage after burns. The primary goal in the management of the wound is to achieve protective infection, rapid healing with optimal functional, decrease morbidity and mortality. This is best accomplished by preventing infection and providing an environment that optimizes healing of the wound.
Pharmacological therapy
Burn wounds are rapidly susceptible to bacterial growth with the potential for invasive infection. A topical antibiotic is commonly used for burn wounds. A topical antibiotic should be applied to prevent and treat infection with second and third degree burns.4,18,19 Silver sulfadiazine (SSD) is the standard treatment used for prophylaxis against infection but is generally not used for superficial burns such sunburn.1,2 It should be applied with a sterile-gloved hand at a thickness of 1/16 inch. The burned area should be covered with cream at all times.20 Treatment with SSD may delay wound healing and increase the frequency of dressing changes, resulting in increased pain.
The action of silver sulfadiazine is driven by inhibition respiratory enzymes and components of the microbial electron transport system, as well as impairing some DNA function. The inhibitory action of silver can be attributed to its strong interaction with thiol groups present in cell respiratory enzymes in the bacterial cell. The mechanism of action is upon the bacterial cell wall and cell membrane.4, 21 Silver sulfadiazine is a bactericidal for many gram-negative, gram-positive bacteria and yeast such as active against; Pseudomonas aeruginosa, Pseudomonas maltophilia, Enterobacter species, Klebsiella species, Serratia species, Escherichia coli, Proteus mirabilis, Morganellamorganii, Providenciarettgeri, Proteus vulgaris, Providencia species, Citrobacter species, Acinetobactercalcoaceticus, Staphylococcus aureus, Staphylococcus epidermidis, Enterococcus species, Candida albicans, Corynebacteriumdiphtheriae, and Clostridium perfringens.20
Pharmacokinetic studied of silver sulfadiazine revealed that there is a concern about the silver absorption via skin from partial- and full-thickness burn wounds (5% body surface area), where most of the silver is associated with area of burn wound surface and very little is absorbed into deeper layers.21 Silver sulfadiazine is metabolized via the liver but the mechanism is still unknown. It has been estimated that half-life elimination is around 10 hours, prolonged by renal impairment, reaching a peak in serum at around 3 - 11 days of continuous therapy as well as excretion through the urine (approximately 50% as unchanged drug).20
Adverse effects of tropical therapy when applied to skin such as skin rashes, especially patient allergy to sulfonamide group It should be avoided patient with hepatic or renal impairment. Previous study has shown that Ag+ in silver sulfadiazine is toxic to liver tissue and the kidneys20,22 and also causes bone marrow toxicity, which leads to abnormalities in blood synthesis, including delayed wound healing.21
Non-pharmacological therapy
Burn wound dressing is an alternative way to assist with the healing of burn wounds. At present, there are many burn wound dressing products that are widely used such as Acticoat, Urgotul SSD, Aquacel Ag+, Mepitel, AskinaCalgitrol Ag+.4,23 All of the above have a good effect on burn wounds if they are suitably used for each degree of burn, especially for superficial and partial thickness burns. In the case of severe burns which range from deep partial thickness burns to the third-degree burns, surgery and skin graft transplantation would yield a better result.
Skin graft transplantation is one of the most popular ways to respond to deep partial burn wounds and third-degree wound healing. This method was used in the 18th century by British surgeons but it was not quite successful in that age. In 1870, George David Polock successfully carried out skin graft transplantation with burn wound patients and this method has been continually developed until now.24
New technologies for burn wound closure and healing
Up to the present, there has been an attempt to develop several techniques and products which could enhance the wound-healing processes in terms of the healing period, and reducing the death rate and also complications, resulting in shorter stays in the hospital and reducing the incapacity rate after wound healing. Thus, there has been the development of wound dressing materials for wound healing process enhancement.
Later, after the development pioneer period, temporary wound dressings used with biological materials were able to control the number of pathogens or covered a significant proportion of blood vessels. This kind of material could reduce the wound size, protecting the wound from pathogens and promoting the wound-healing processes. After the wound gets better, the physician can further consider or search for other recovery methods in order to recover the organ function or to obtain a good appearance later on, for example, with large burn wounds or necrotizing fasciitis. As in the case of biological dressing, human allograft (cadaver skin) or xenograft (e.g. pig skin) could be used in burn wound dressing. Allograft might be accompanied with the biological dressing but only at around 5-10 days after the dressing, depending on the immune system of the recipient.25
Today, there is the development of silicone polymers or composite membranes by using skin graft theory. This material could be used for temporary wound covering. Nowadays, scientists have developed tissue cultures in order to be used as skin structure, resulting in better healing results; however, research is still in the development phases as with other techniques, such as dermal regeneration templates (artificial skin substitutes), dermal culture, fibroblast cultures, including cytokine and gene therapy.23
Wound healing is a complex process of repairing after tissue injury. Burn is one of the most common types of injuries. SSD cream is the gold standard treatment used for preventing bacterial gram positive and gram negative growth in the burn wound and for wound healing. Nowadays, there are many new burn wound dressing products that are widely used but SSD cream is still more popular than new products, as SSD cream is a low cost product and shows good benefits. It is essential that the healthcare provider understands the importance of the phathophysiological processes, and good management. This will reduce morbidity and mortality in wound care.
Q1. Which of the following is not true of wound-healing process?
a. Wound healing has three phases.
b. Hemostasis phase is the first phase of wound healing.
c. Inflammatory phase is the second phase of wound healing.
d. Proliferation phase is the third phase of wound healing.
Q2. What is the first phase of wound healing?
a. Inflammatory phase
b. Hemostasis phase
c. Maturation or remodeling phase
d. Proliferation phase
Q3. What is the method use to assessment of the severity area of burn wounds?
a. Palmer surface area
b. William’s rule of nines
c. Lund and Browdermethod
d. All of the above
Q4. What is the gold standard treatment of wound?
a. Silver chloride
b. Silver nitrate
c. Silver nitrite
d. Silver sulfadiazine
Q5. What is the new technologies for burn wound closure and healing?
a. Silver sulfadiazine cream
b. Thai herbals
c. Tissue cultures
d. All of the above
Answers 1: a. Wound healing has four phases: hemostasis, inflammation, tissue proliferation and tissue maturation or remodeling that overlap in time.
Answers 2: b. Hemostasis phase is the first phase of wound healing process.
Answers 3: d. Palmer surface area, William’s rule of nines, and Lund and Browder method are used to assessment of the severity area of burn wounds.
Answers 4: d. Silver sulfadiazine cream is gold standard treatment used for preventing bacterial gram positive and gram negative growth in the burn wound and for wound healing.
Answers 5: c. Tissue cultures such as dermal culture, fibroblast cultures, including cytokine and gene therapy are new technologies for burn wound closure and healing.